Novel synthetic c-glycolipids, their synthesis and use to treat infections, cancer and autoimmune diseases
A disease and glycolipid technology, applied in the field of new synthetic C-glycolipids, which can solve the problems of reducing in vivo efficacy and short half-life of α-GalCer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
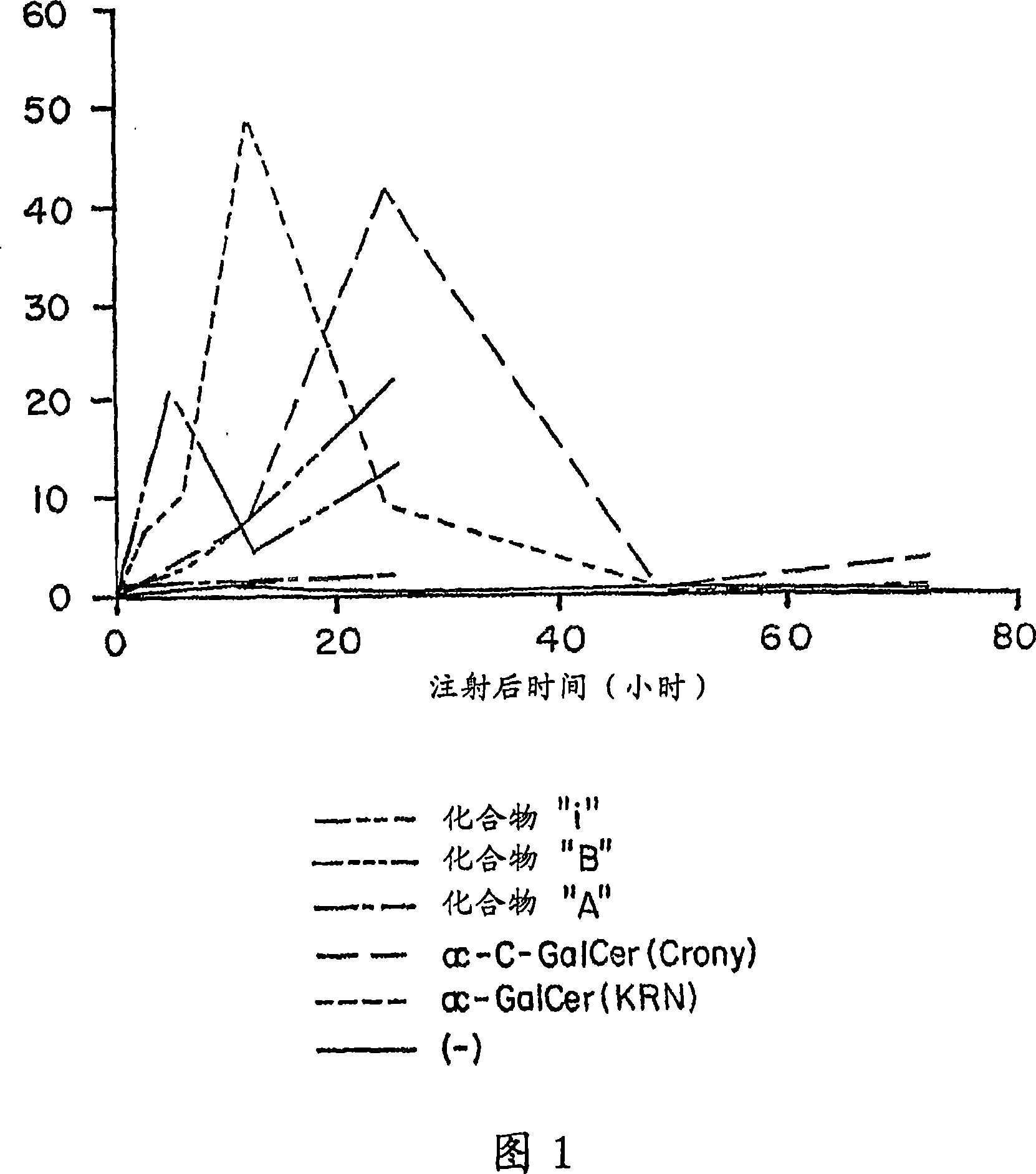
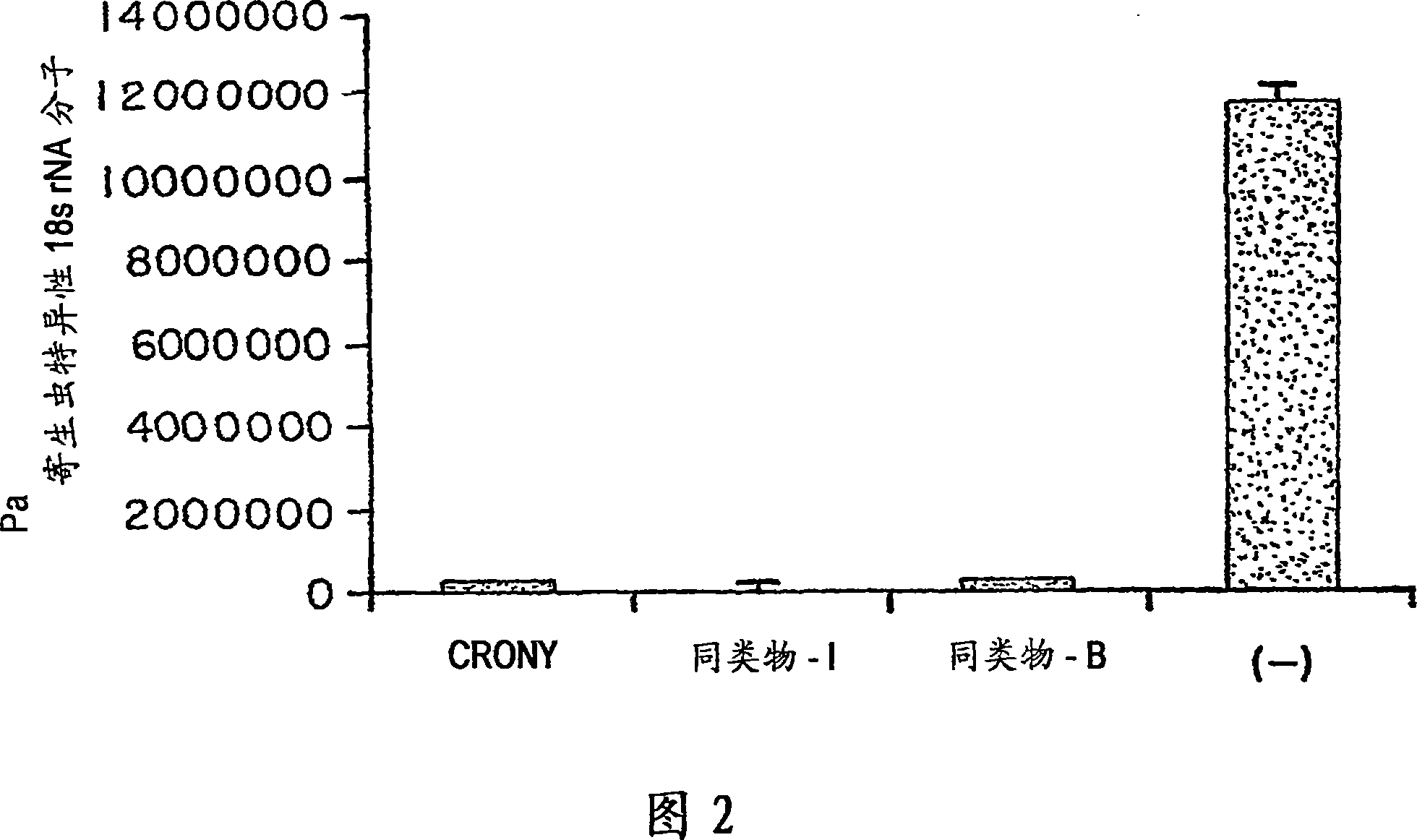
Image
Examples
Embodiment
[0337] A further understanding of the compounds of the invention, their preparation, and methods of use may be provided by the examples illustrating some of the methods of making or using these compounds. These examples do not limit the invention. Variations of the invention known or further developed are considered to fall within the scope of the invention, as hereinafter claimed.
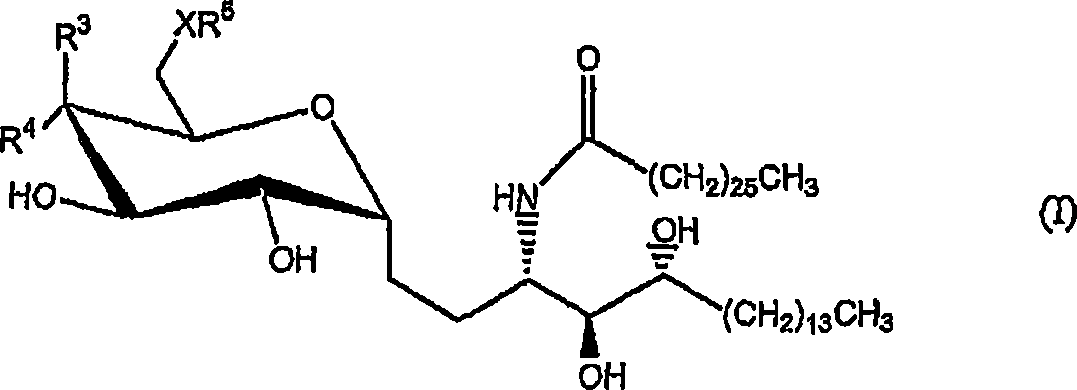
[0338] Compounds and Chemical Synthesis
[0339]
Embodiment A
[0340] Example A: Y 5 : Preparation of CBZ or tBoc
[0341] The reaction process proceeds as follows:
[0342]
[0343] condition:
[0344] Step (a):
[0345] For benzyl carbamate: CBZCl (1.1 eq.), 1N NaHCO 3 , 1,4-dioxane, ethyl acetate, rt (room temperature), overnight, the yield was 90%;
[0346] For tert-butyl carbamate: 1N NaOH (1.5 eq.), (t-Boc) 2 O (1.5 eq.), ethanol, water, rt, 1h;
[0347] Step (b):
[0348] For benzyl carbamate: TBSCl (tert-butyldimethylsilyl chloride) (1.2 eq.), Et 3 N (1.1 eq.), 4-DMAP (4-dimethylaminopyridine) (0.05 eq.), DCM (dichloromethane), DMF (dimethylformamide), 0°C, 1h, 96%;
[0349] For tert-butyl carbamate: The overall yield of steps (a) and (b) was 93%.
[0350] Step (c):
[0351] For benzyl carbamate: 2,2-dimethoxypropane (5-10 eq.), PPTs (pyridinium p-toluenesulfonate) (0.07 eq.), DCM, rt, 2h, yield 99% ;
[0352] For tert-butyl carbamate: The reaction product was used directly in the next step without purification.
[0...
Embodiment B
[0362] Example B: Y 1 , Y 2 , Y 3 and Y 4 Each independently is Ac or Bn; n=1 or 0 preparation
[0363] The reaction process proceeds as follows:
[0364]
[0365] condition:
[0366] Step (c):
[0367] o 3 , DCM, -78°C;
[0368] Step (d):
[0369] NaBH 4 , DCM, MeOH, the yield is 40% (three steps);
[0370] Step (e):
[0371] Allyltrimethylsilane (3.0 eq.), BF 3 .OEt 2 (5.0 eq.), 0-10 ° C, 3 days, the yield is 77%;
[0372] Step (f):
[0373] NaOMe (0.1 eq.), MeOH, rt, 1h;
[0374] Step (g):
[0375] NaH (2.0 eq.), BnBr (1.5 eq.), TBAI (catalytic amount), DMF, THF, rt, 14h, the yield is 93% (2 steps);
[0376] Step (h):
[0377] PdCl 2 (PhCN) 2 , benzene, reflux, 20h, yield is 73%; (i) (COCl) 2 (2.25 eq.), DMSO (5.50 eq.), DCM, -78°C, 0.5-1h, then Et 3 N (6.0eq.), to 0°C, 2h, the yield was 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com