Preparation method of tumor vaccine and tumor vaccine prepared by using the method
A tumor vaccine and tumor antigen technology, applied in the field of cellular immunotherapy, can solve the problems of low immune response level and high adjuvant dependence of tumor vaccines, achieve strong immune stimulating activity, improve screening accuracy, and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The invention discloses a method for preparing a tumor antigen and the tumor antigen prepared by using the method. Those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. It needs to be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention, and relevant personnel can obviously make changes without departing from the content, spirit and scope of the present invention. Changes or appropriate changes and combinations are made to the content described herein to realize and apply the technology of the present invention.
[0055] In the present invention, unless otherwise specified, the scientific and technical terms used herein have the meanings commonly understood by those skilled in the art.
Embodiment 1
[0057] Example 1: Design of the vaccine
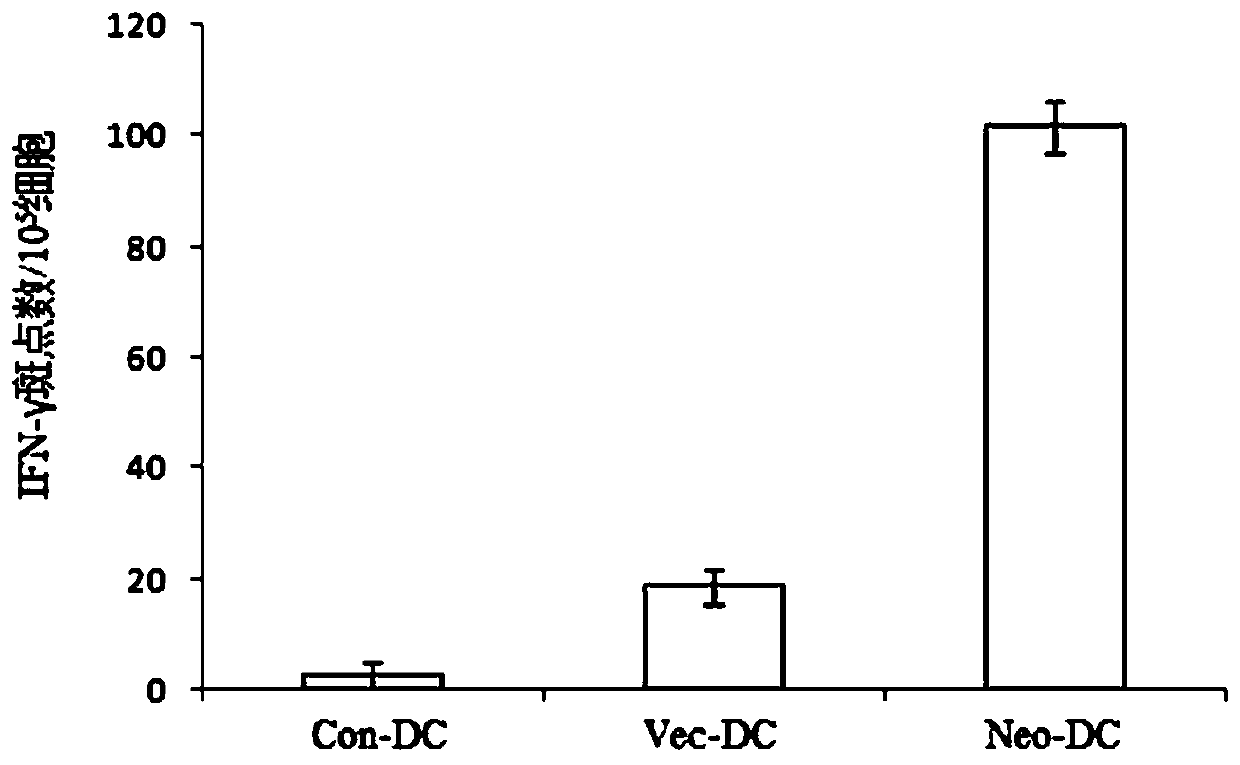
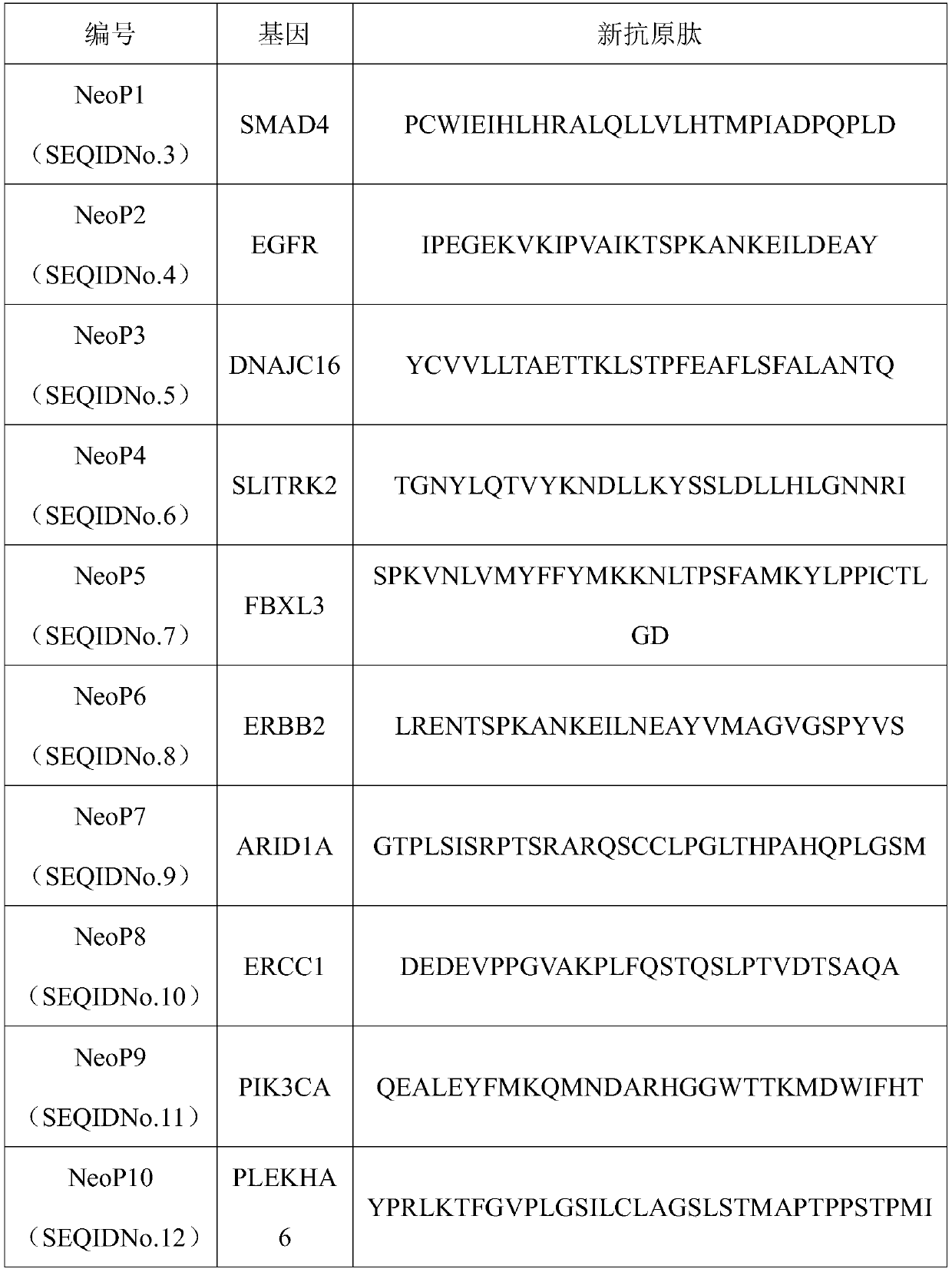
[0058] The tumor tissue and peripheral blood of tumor patients were collected, exome DNA sequencing was performed on tumor tissue and peripheral blood leukocytes, and RNA sequencing was performed on tumor tissue. Sequencing data is analyzed to identify tumor-specific non-synonymous mutations. Antigen prediction software NetMHCpan 4.0 and NetMHCIIpan 3.2 were used to predict the affinities of neoantigens containing non-synonymous mutations to MHCI and MHCII respectively. The stronger the binding ability. Select neoantigen peptides with strong affinity and significantly higher than wild-type antigen peptides as candidate tumor neoantigen peptides, which can effectively stimulate the immune response and have little effect on normal cells expressing wild-type antigen peptides , the candidate tumor neoantigen peptides are shown in Table 1.
[0059] Table 1 Candidate tumor neoantigen peptides
[0060]
Embodiment 2
[0061] Example 2: Construction and expression of vaccines
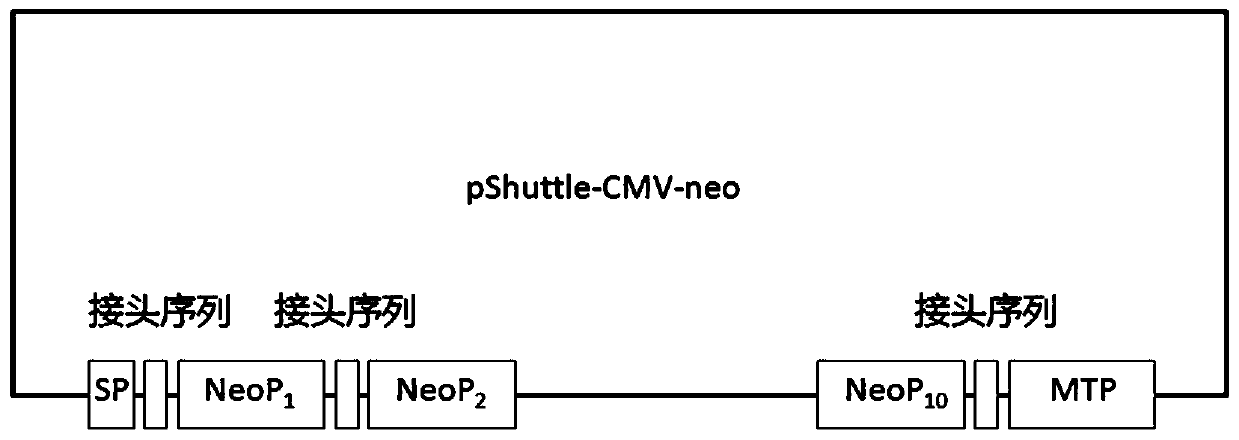
[0062] Synthesize the DNA sequence corresponding to the neoantigen peptide and clone it into the pShuttle-CMV plasmid, such as figure 1 As shown, pShuttle-CMV-neo was obtained, and finally verified by DNA sequencing. Transfer pShuttle-CMV-neo into BJ5183 competent cells containing pAdEasy, pick a single clone and identify it, obtain the recombinant adenovirus plasmid pAdEasy-pShuttle-CMV-neo that restores the new antigen peptide, and construct the empty recombinant adenovirus plasmid pAdEasy at the same time -pShuttle-CMV. HEK293 cells were transfected to obtain recombinant adenovirus Ad-neo and empty recombinant adenovirus Ad-Vec.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com