Compound I and compound II as well as preparation methods and application thereof
A compound and reaction technology, applied in the field of compound I and compound II and their preparation, can solve problems such as poor therapeutic effect and adverse reactions, and achieve the effect of slowing down growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
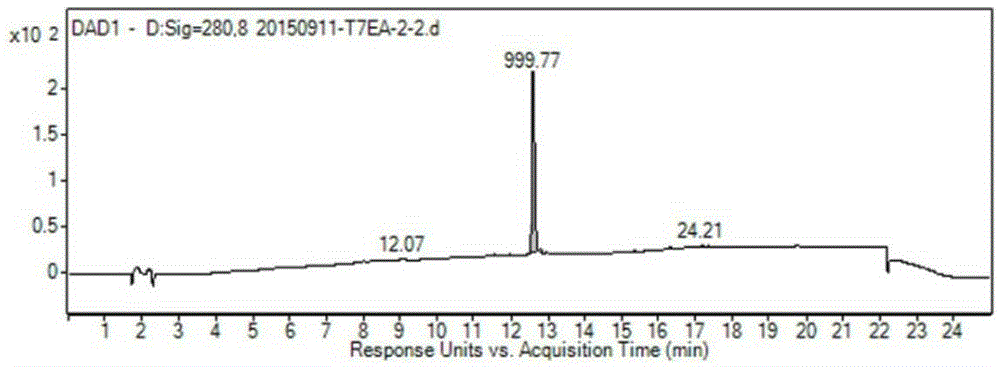
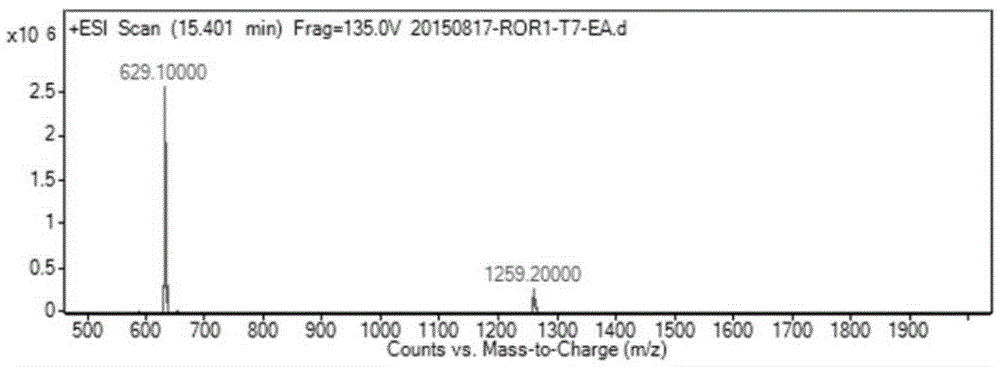
[0049] Weigh 344 mg (1 mmol) T7 and 341 mg (1.125 mmol) EA and dissolve in 8 mL DMF, add HBTU (427 mg, 1.125 mmol), triethylamine (416 μL, 3 mmol) and a catalytic amount of DMAP, and stir the reaction at room temperature for 12 hours. The reaction solution was poured into 100 mL of water, filtered with suction, and the filter residue was washed with water and dried to obtain a crude product. After separation by column chromatography (DCM:MeOH=20:1), 475 mg of T7-EA was obtained as a white solid with a yield of 75.5%. Purity: 95.08%, ESI-MS: m / z=629.1[M+H] + .(Such as Figure 1-2 shown)
preparation Embodiment 2
[0051] Weigh 172mg (0.5mmol) of T7 and 189.4mg (0.625mmol) of EA and dissolve in 6mL of a mixed solvent of benzene and toluene with a volume ratio of 2:1, add HBTU (237.2mg, 0.625mmol), triethylamine (312μL , 2.25mmol) and a catalytic amount of DMAP, stirred at room temperature for 24 hours. The reaction solution was poured into 100 mL of water, filtered with suction, and the filter residue was washed with water and dried to obtain a crude product. After separation by column chromatography (DCM:MeOH=20:1), 236.8 mg of T7-EA was obtained as a white solid, with a yield of 73.2%.
preparation Embodiment 3
[0053] Weigh 516mg (1.5mmol) of T7 and 568.3mg (1.875mmol) of EA and dissolve in 15mL of tetrahydrofuran, add HBTU (711.7mg, 1.875mmol), triethylamine (520μL, 3.75mmol) and a catalytic amount of DMAP, and stir at room temperature React for 18 hours. The reaction solution was poured into 200 mL of water, filtered with suction, and the filter residue was washed with water and dried to obtain a crude product. After separation by column chromatography (DCM:MeOH=20:1), 752 mg of T7-EA was obtained as a white solid, with a yield of 77.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com