Live bacterial vaccines resistant to carbon dioxide (CO2), acidic ph and/or osmolarity for viral infection prophylaxis or treatment
a technology of carbon dioxide and bacterial vaccines, applied in the field of live bacterial vaccines for viral infection prophylaxis or treatment, can solve the problems of reducing the effectiveness of vaccines not matched to the new variant, inability to achieve adequate timescale, and ineffectiveness of oseltamivir (tamiflu®) in 50% of cases, so as to achieve the effect of raising the immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
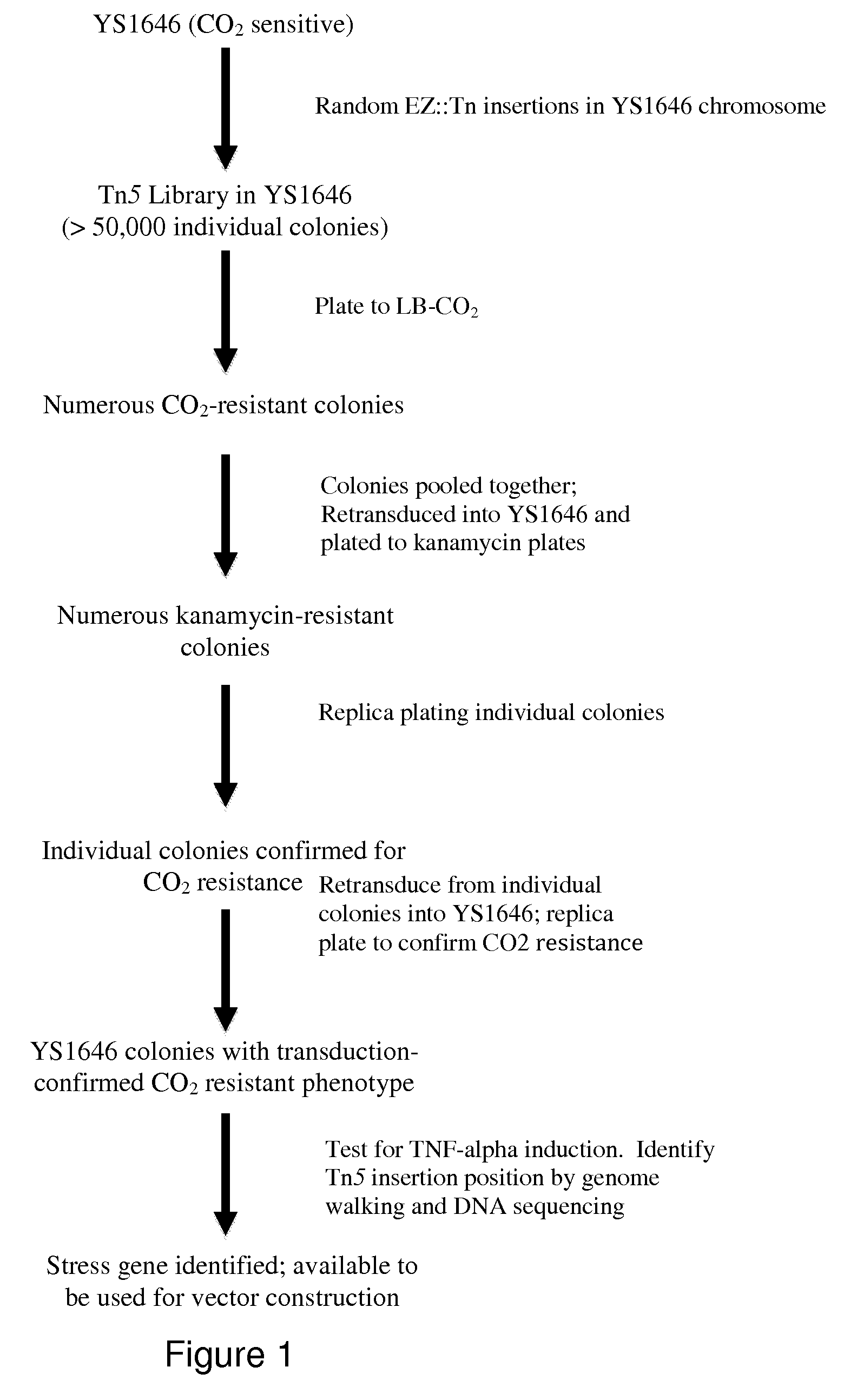
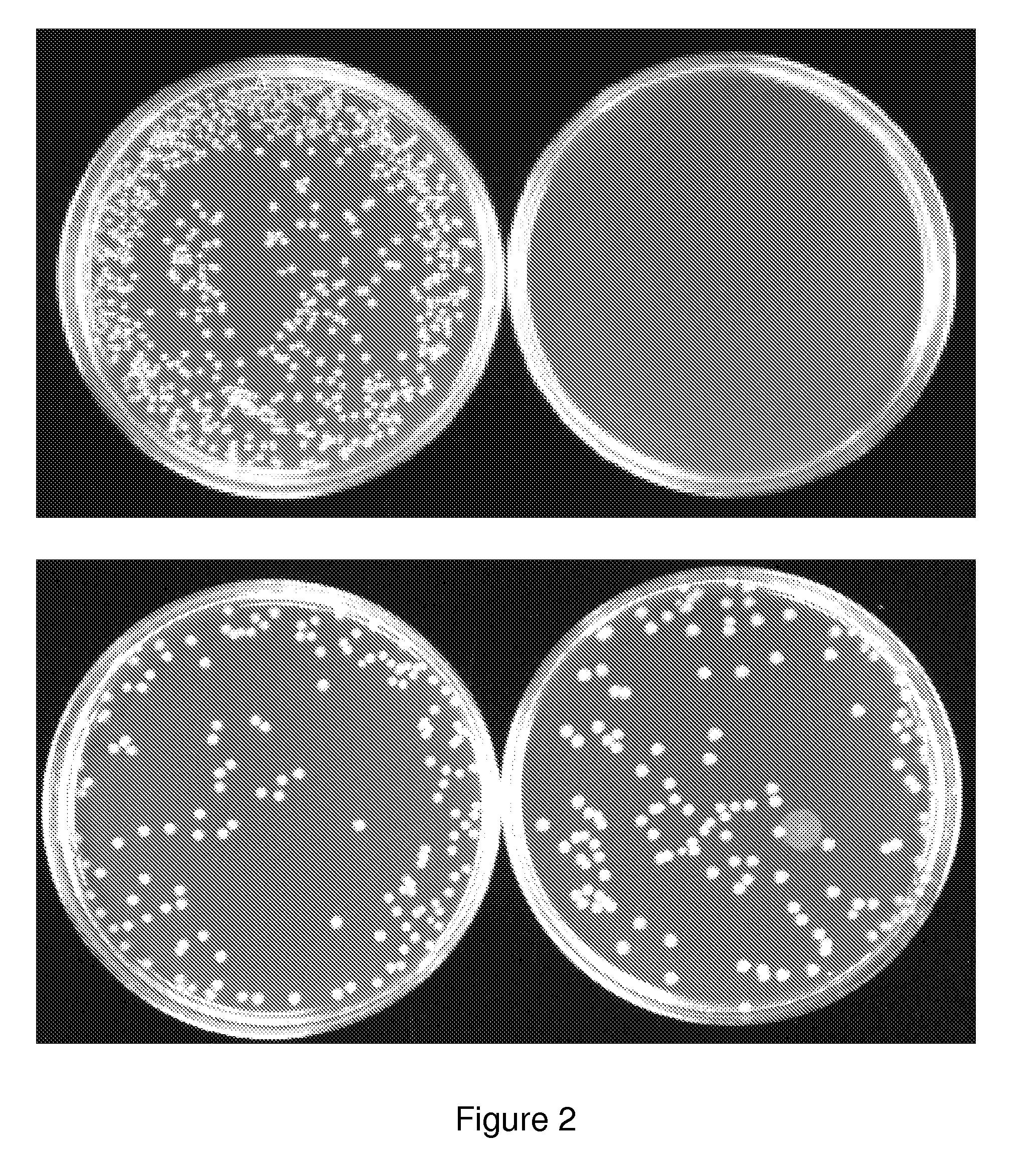
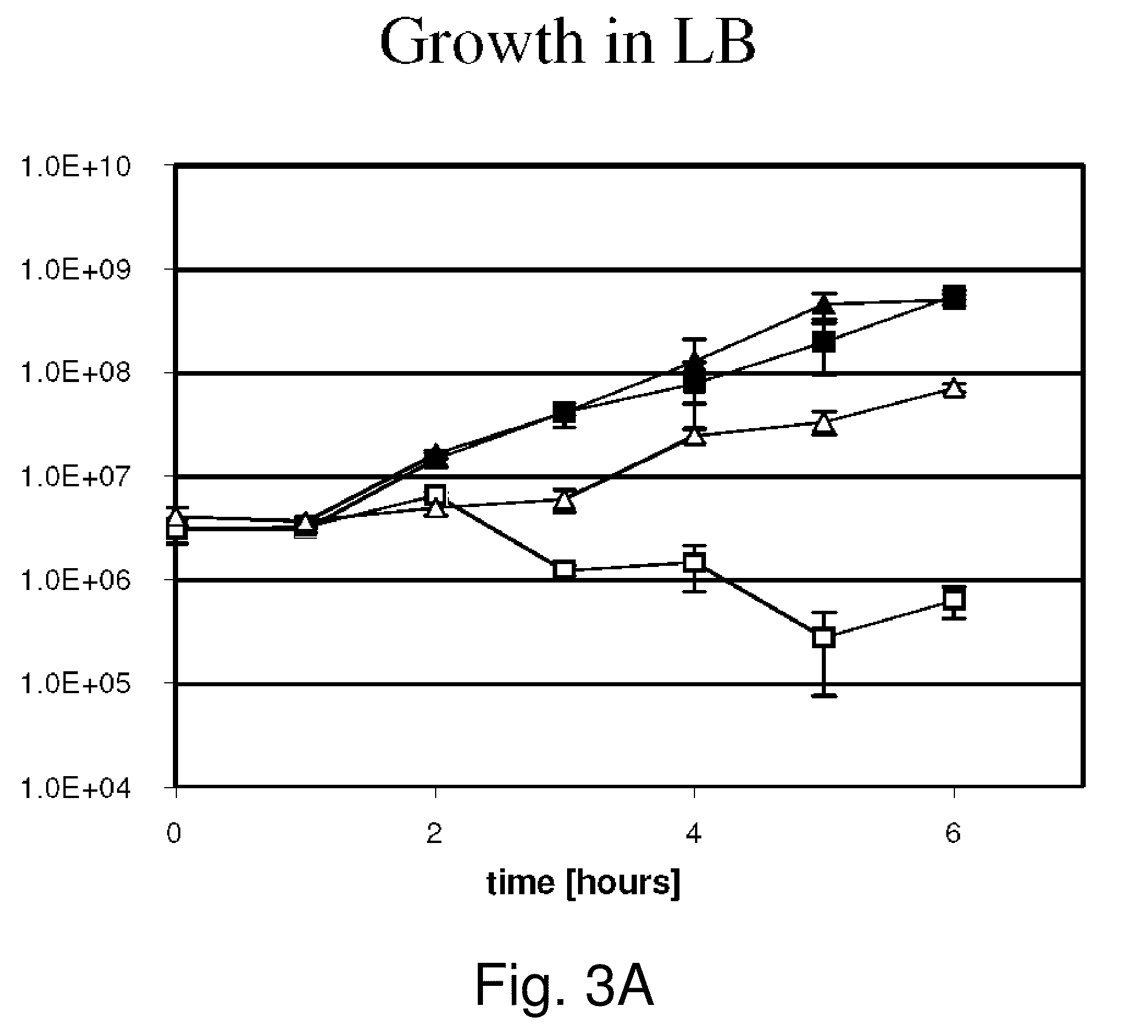
[0098]The invention provides gram-negative bacterial mutants resistant to one or more stress conditions, including, but not limited to, CO2, acid pH, and / or high osmolarity. In one embodiment, the present invention provides gram-negative bacterial mutants resistant to CO2, acid pH, and / or high osmolarity. In a more preferred embodiment, the present invention provides attenuated gram-negative bacterial mutants resistant to CO2, acid pH, and / or high osmolarity. Preferably, the stress-resistant gram-negative bacterial mutants are attenuated by introducing one or more mutations in one or more genes in the lipopolysaccharide (LPS) biosynthetic pathway that reduces the induction of TNF-α, and optionally, one or more mutations to auxotrophy for one or more nutrients or metabolites.
[0099]The invention also provides stress-resistant gram-negative bacterial mutants engineered to contain and / or express one or more nucleic acid molecules encoding one or more therapeutic molecules. In a specific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Osmolarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com