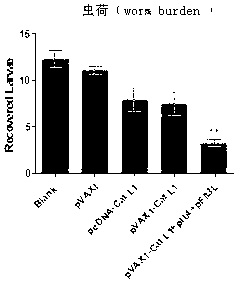
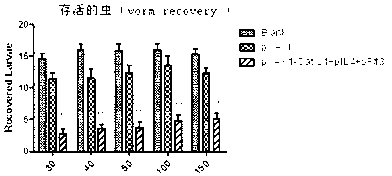
Vaccine composition for improving immune protective rate of anti-Fasciola hepatica Cat L1 DNA, and preparation method thereof
A technology of DNA vaccine and Fasciola hepatica, which is applied in the field of vaccine combination and preparation to improve the immune protection rate of DNA vaccine against Fasciola hepatica, achieving high efficiency, easy operation and strong pertinence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] 3. Preparation of Cat L1 polyclonal antibody
[0044] Five 6-7-week-old female BALB / c mice were injected intramuscularly with 40 μg / mouse of purified Cat L1 protein in the hind legs, and immunized for a total of 3 times, each time at an interval of 1 week, and blood was collected 1 week after the last immunization Separate the serum for later use. Antibody concentration was determined by Coomasie protein assay.
[0045] 4. Identification of target protein by Western blotting
[0046] The protein purified by the His-Tag fusion protein purification kit in method 2 was electrophoresed on SDS-PAGE (30V, 1h) and then transferred to a nylon membrane for Western blotting identification. Wash the membrane twice with 15ml 1×TBS, then add 5% non-fat milk powder TBS solution to block unbound protein sites. After washing twice with 1×TTBS, incubate the nylon membrane in polyclonal antibody for 1 hour, use the obtained Cat L1 polyclonal serum (diluted at 1:300), wash with 1×TTBS ...
specific Embodiment
[0061] The present invention is mainly divided into two major plates. One is to design and construct the required plasmids, and the other is to screen and evaluate vaccine combinations. The technology of the present invention will be described in detail below in conjunction with the accompanying drawings:
[0062] 1. Design and construct the required plasmid
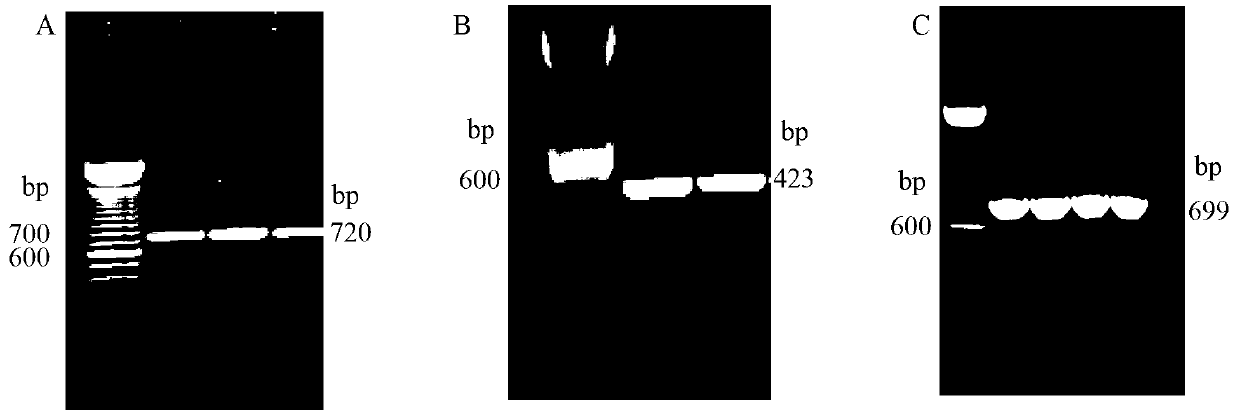
[0063] First, in order to obtain the candidate vaccine gene FhCat L1, fresh worms obtained from bovine liver were used to extract mRNA (see QIAGEN mRNA kit for specific extraction methods). Then obtain the cDNA of FhCat L1 gene and the required ORF fragment with RT-PCR method, and connect it in the pMD-18 cloning vector, form pMD-Cat L1 plasmid, then carry out PCR sequencing identification (see figure 1 ), which is exactly in line with the expected results. IL4 and Flt3L were cloned directly from the peripheral blood of mice, and the method used was carried out according to the whole blood mRNA kit. Then, the two ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com