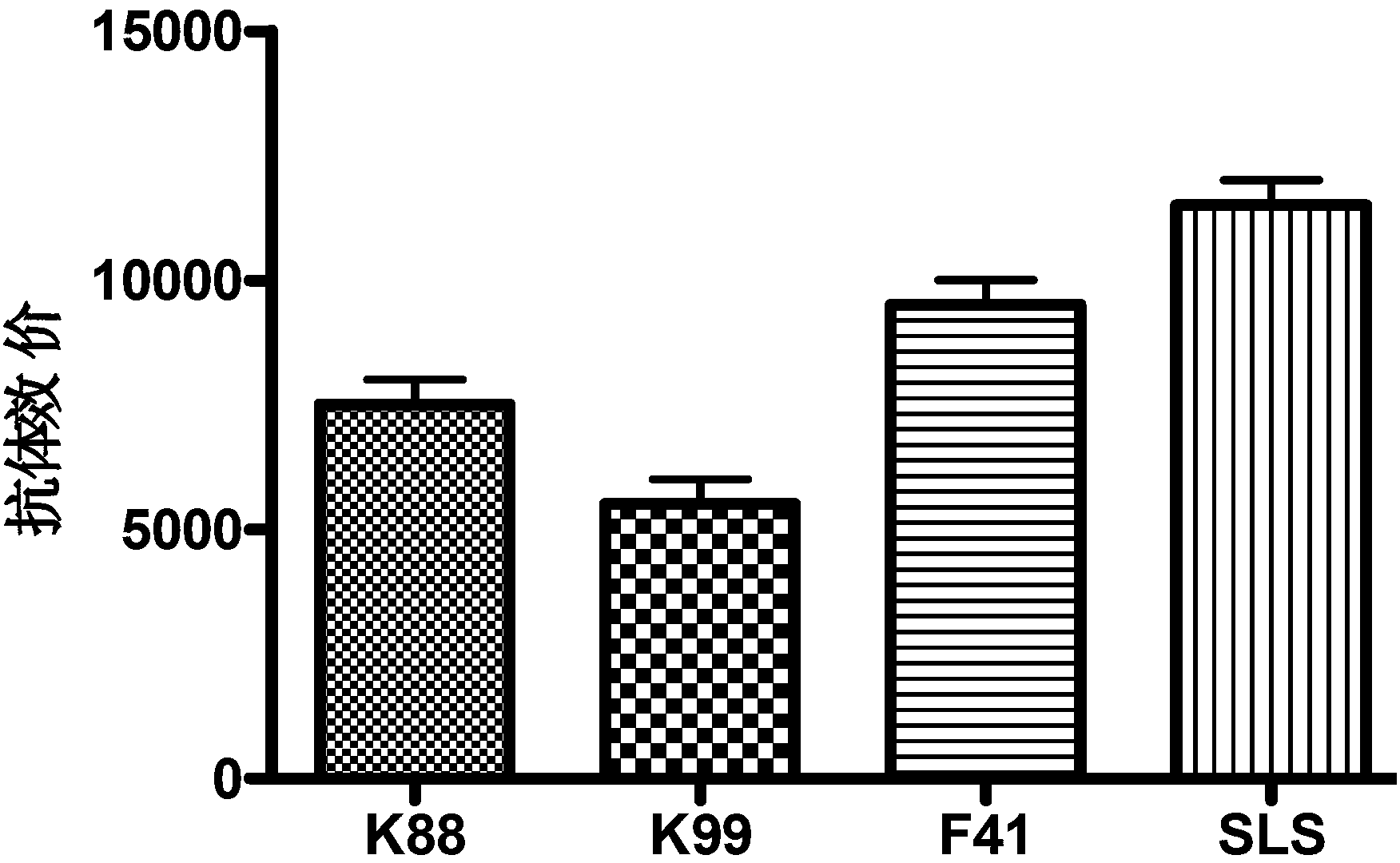Recombined enterotoxin (namely, fimbriae composite polyvalent vaccine) for resisting diarrhea of piglets and preparation method for recombined enterotoxin (namely, fimbriae composite polyvalent vaccine)
A technology for piglet diarrhea and multivalent vaccines, applied in antibacterial drugs, pharmaceutical formulas, bacterial antigen components, etc., can solve the problems of geographical restrictions on vaccine use, single antigen, and low immune protection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Specific embodiments of the present invention will be described in detail below in conjunction with technical solutions and accompanying drawings.
[0029] 1. Vaccine preparation
[0030] 1. Primary strain culture and strain identification of standard pili strains
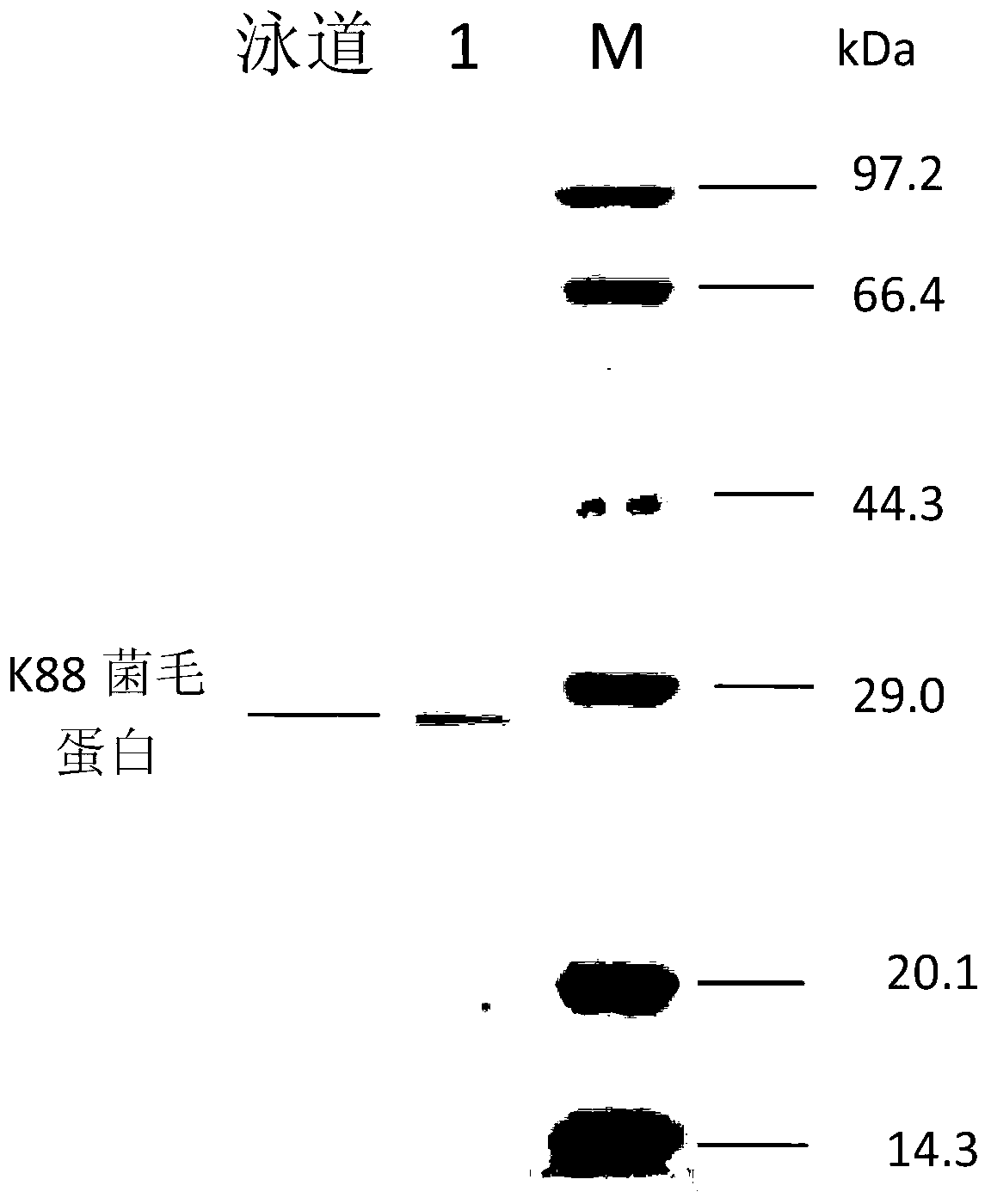
[0031] The standard K88 pili, K99 pili and F41 pili strains were inoculated on the modified Minca medium, cultured overnight at 37°C, and blood agglutination experiments were carried out with the corresponding (K88, K99, F41) pili antiserum, and the agglutination reaction reached " ++++", the pure inspection is qualified. The bacterial solution was added to 50% glycerol and frozen in a -70°C refrigerator.
[0032] 2. Secondary strain culture and extraction and identification of antigenic protein
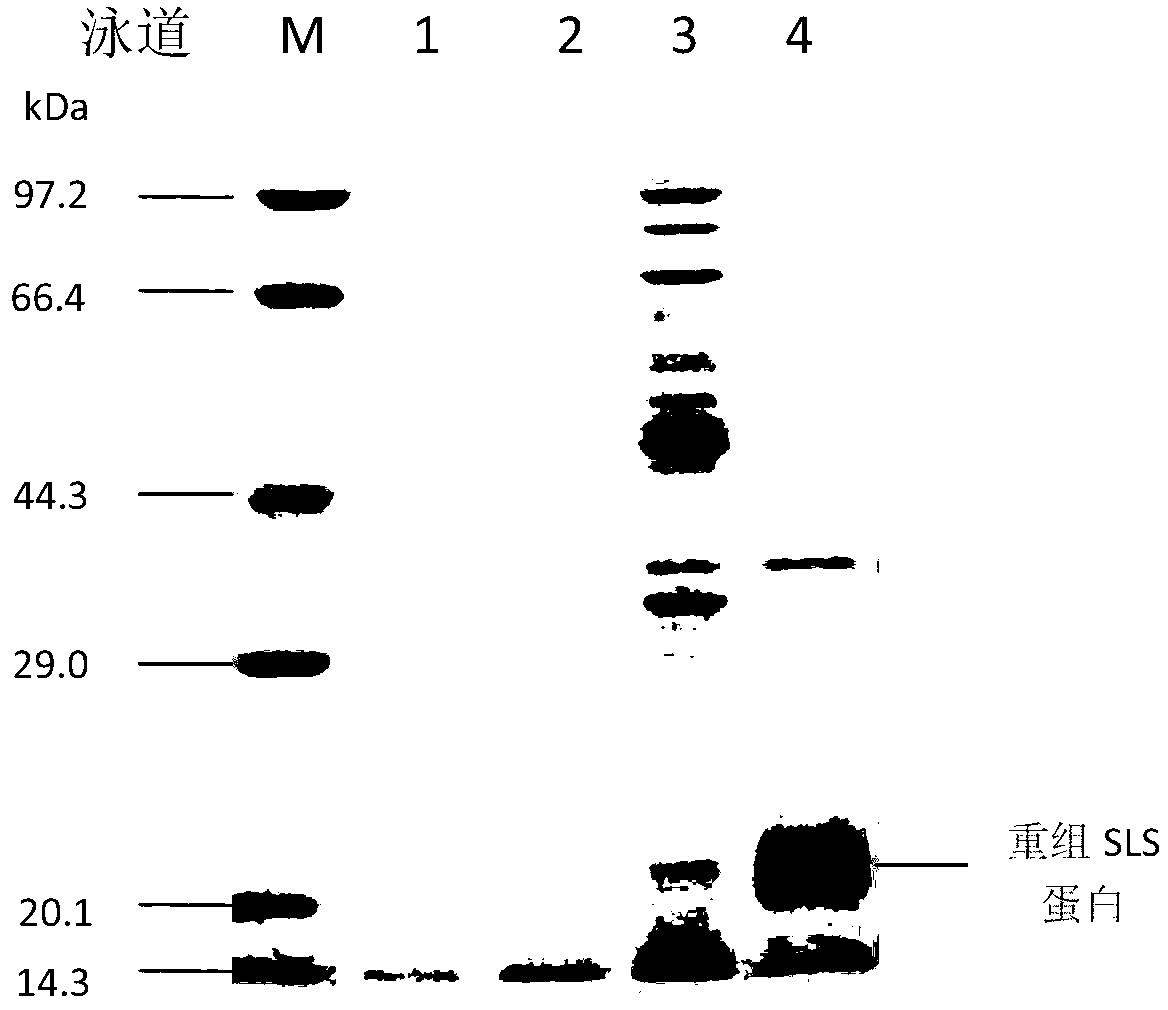
[0033] (1) Extraction of recombinant enterotoxin protein SLS
[0034] The trivalent enterotoxin recombinant bacterium pET30-SLS was inoculated in TSB medium and cultured with shaking at 37°C. Microplate reader...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com