Oncolytic rhabdovirus expressing il12
A virus and oncolytic technology, applied in the field of recombinant oncolytic rhabdovirus, can solve the problem of reducing effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
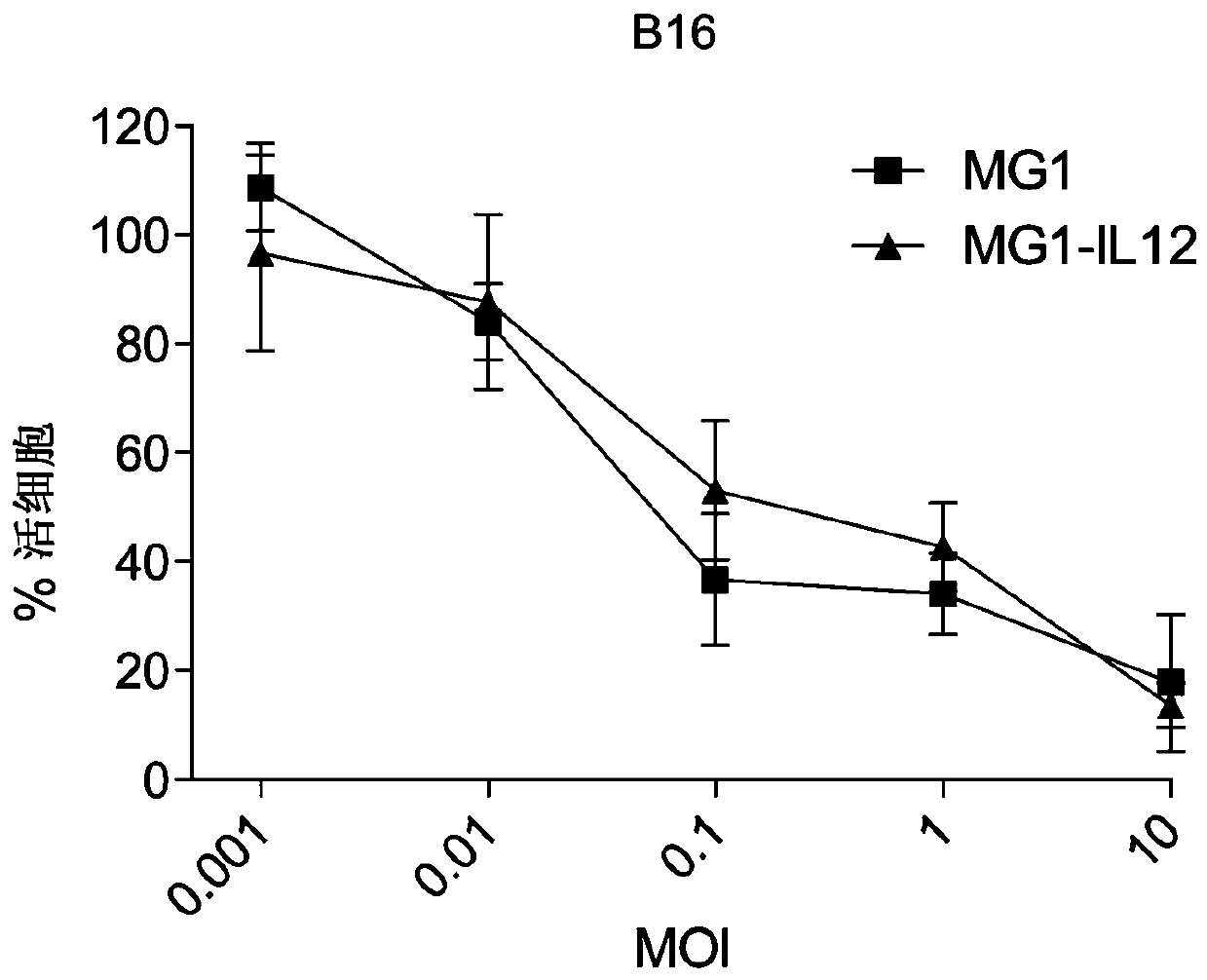
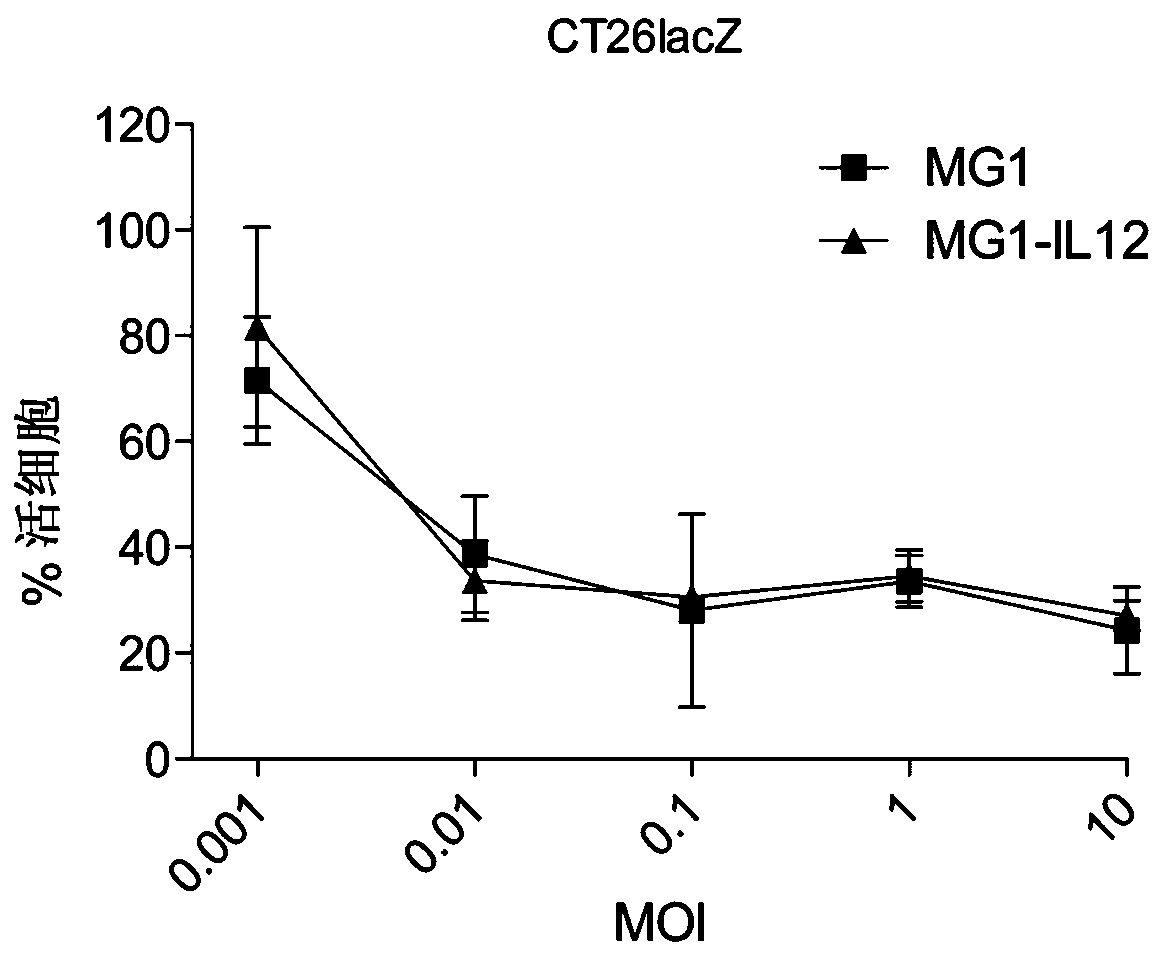
[0101] MG1-IL12 ICV enhances NK cell-mediated tumor rejection.
[0102] The authors of the present disclosure have previously demonstrated that ex vivo infection of autologous tumor cells with an oncolytic virus can elicit a robust immune response against established, non-permissive tumors in vivo (15). To determine whether MG1 and MG1-IL12 could similarly induce immune responses when used as ICV, the authors injected intravenously (i.v.) 5x10 5 Ɣ-irradiated B16F10 cells that were either mock-infected or infected with MG1 or MG1-IL12. The authors have previously demonstrated that intravenous administration of ICV is associated with rapid and dose-dependent accumulation of injected cells in the lung lasting up to 1 day in tumor-free animals (16). After ICV delivery, significantly higher levels of IL12 were detected in lung homogenates of mice receiving MGI-IL12 ICV compared with animals receiving ICV of cells alone or MG1 ( Figure 6 , t = 24 hours). To determine whether in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com