Chemiluminiscence kit for detecting anti-envelope glycoprotein antibody in serum of HCV infected person, and detection method
A chemiluminescence reagent and envelope glycoprotein technology, which is applied in chemiluminescence/bioluminescence, biological testing, and analysis through chemical reactions of materials, etc., can solve the problems of inability to distinguish antibodies, inability to receive effective treatment, and exacerbation of disease prevalence, etc. problem, to achieve high-sensitivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] specific implementation plan
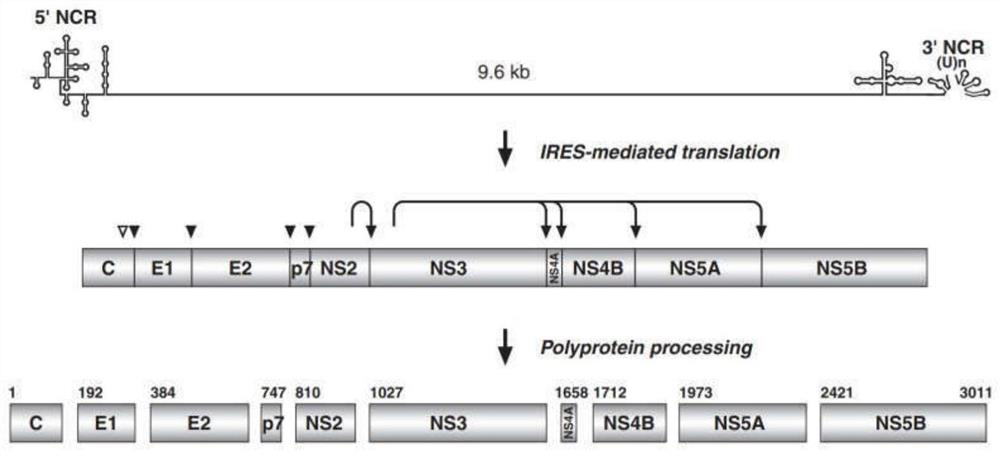
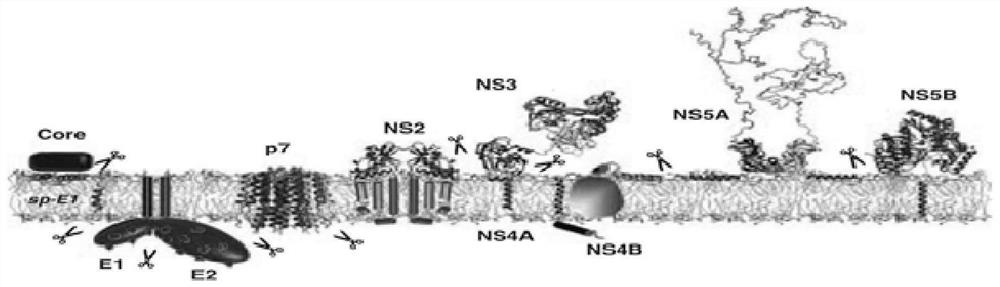
[0037] The genome of hepatitis C virus HCV is a single-stranded positive-strand RNA with a total length of about 9.6kb. There are 7 genotypes (1-7) and 67 genotypes. In my country, genotypes 1b and 2a are common. The HCV genome consists of The 5' non-coding region (5'-NCR) at both ends, the 3' non-coding region (3'-NCR) and an open reading frame (ORF) in the middle, the ORF is immediately downstream of the 5'-NCR, and its genome arrangement The sequence is 5'-C-E1-E2-NS1-NS2-NS3-NS4-NS5-3', which can encode a polyprotein precursor consisting of 3010-3030 amino acids, such as figure 1 shown. Under the action of the host cell signal peptidase and the virus's own protease, cleavage produces 10 kinds of HCV proteins, such as figure 2 As shown, including: 3 kinds of structural proteins, namely nucleocapsid protein (Core), envelope glycoprotein (E1, E2), transmembrane protein (P7); 6 kinds of nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| intra-assay coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com