Vaccine composition and preparation method and application thereof
A vaccine composition and the technology of the composition are applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antibody medical ingredients, etc., and can solve problems such as occasional allergies and transient fever
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
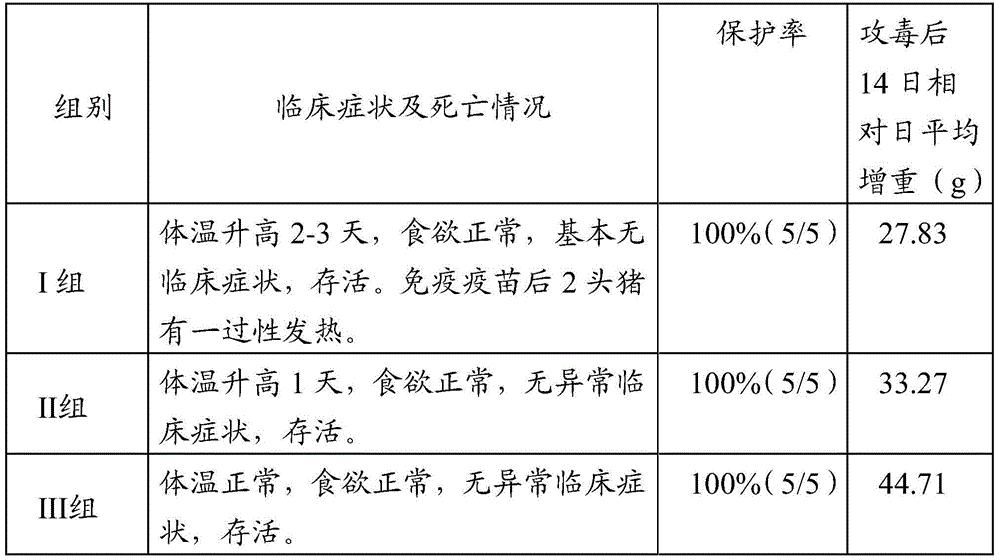
[0077] Example 1 Preparation of porcine pseudorabies antigen
[0078] 1.1 Preparation of Porcine Pseudorabies Antigen by Spinner Bottle Preparation Process
[0079] The porcine pseudorabies virus gE deletion strain was inoculated into PK-15 cell culture to form a seed batch, and then 1% (V / V) of the virus medium was inoculated into the monolayer PK cell culture, and rotated at 37°C Culture, when the lesion reaches 80%, harvest the toxic cell culture medium, after 2 freeze-thaw, collect the poison, and determine the poison value as 10 8.7 TCID 50 / ml. Add 10% (v / v) formaldehyde solution to the virus solution to make the final concentration of formaldehyde 0.2% (V / V), inactivate at 37°C for 18 hours, and stir every 4 hours for 10 min each time.
[0080] 1.2 Preparation of porcine pseudorabies antigen by suspension culture
[0081] The BHK-21 passaged cell line cryopreserved in liquid nitrogen has a bottom area of 75cm 2 The static culture in the square flask of BHK-21 sus...
Embodiment 2
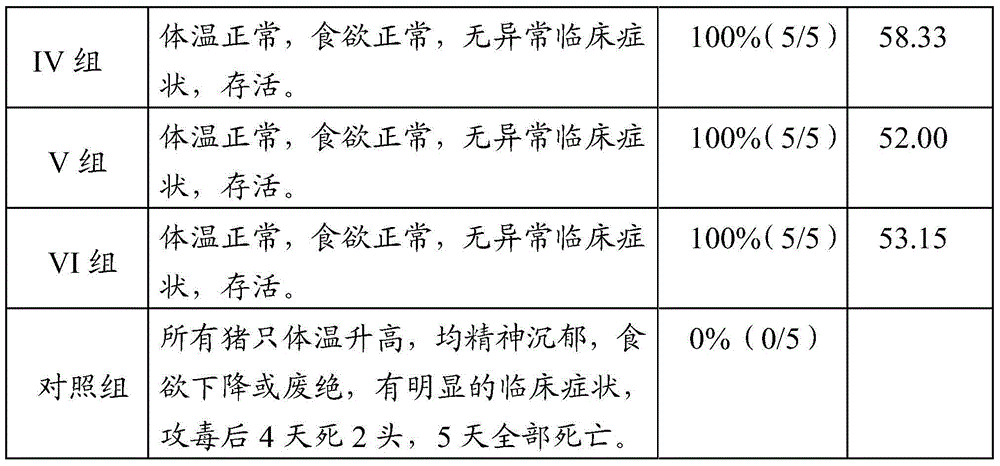
[0082] Example 2 Purification of porcine pseudorabies virus
[0083] 2.1 Preparation of porcine pseudorabies purified antigen: Add 100 ml of the inactivated porcine pseudorabies antigen prepared in Example 1.2 to Triton X114 in a volume ratio of 1.0% (volume ratio) and mix well; put it at 0°C-5°C and mix for 60 minutes , and centrifuged at 37°C + 0.5°C to separate Triton X114 and virus particles. The supernatant is the purified pseudorabies virus antigen. The purified antigen of porcine pseudorabies virus is used as a standard curve with bovine serum albumin. The purified protein content is 53 μg / ml by calculating the protein content at wavelengths of 280 nm and 260 nm.
[0084] 2.2 Preparation of porcine pseudorabies purified antigen: add 100 ml of the inactivated culture of porcine pseudorabies prepared in 1.2 to NP-40 with a volume ratio of 2.0% (volume ratio) and mix well; NP-40 and virus particles were isolated by centrifugation at 37°C + 0.5°C. The supernatant was take...
Embodiment 3
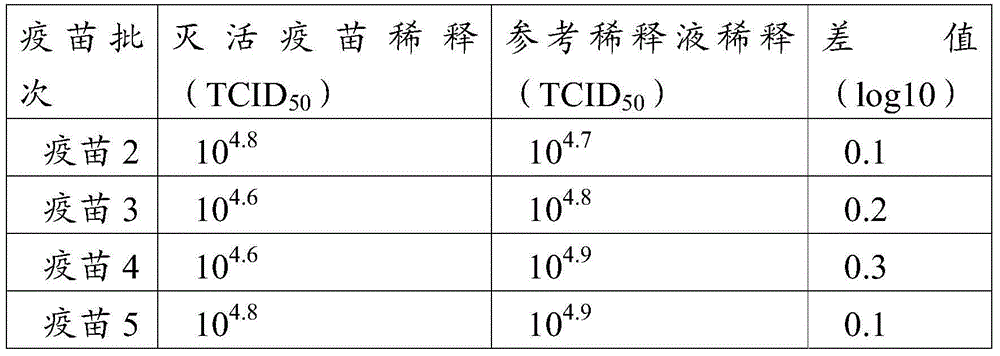
[0088] Example 3 Preparation in porcine pseudorabies vaccine
[0089] Vaccine 1: The porcine pseudorabies virus inactivated antigen prepared in Example 1.1 was mixed with 206 adjuvant (product of French SEPPIC company) in a volume ratio of 54:46, and stirred at 120 rpm for 15 minutes at 30°C.
[0090] Vaccine 2: The purified porcine pseudorabies virus antigen prepared in Example 2.1 was diluted with PBS with a pH of 7.2 to a final vaccine with a protein concentration of 10 μg / head and 206 adjuvant (product of French SEPPIC) according to the volume ratio of 54:46 Mix and stir at 120 rpm for 15 minutes at 30°C.
[0091]Vaccine 3: The purified porcine pseudorabies virus antigen prepared in Example 2.1 was diluted with PBS with a P pH of 7.2 to the final vaccine protein concentration of 10 μg / head. Quil A solution was added to make the final concentration of Quil A 0.1 mg / head. Add cholesterol solution to make the final concentration of cholesterol 0.2mg / head portion, lecithin 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com