Method for rapidly, accurately and repeatedly determining foot-and-mouth disease vaccine antigen 146S
A foot-and-mouth disease vaccine and antigen technology, applied in the field of quantitative analysis of the foot-and-mouth disease vaccine antigen 146S, can solve the problems of undetectable antigens, and achieve the effects of improving quality control, accurate test results, and wide practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 foot-and-mouth disease antigen 146S standard item
[0030] 1. Materials
[0031] (1) Test sample: Type O foot-and-mouth disease inactivated virus liquid comes from Lanzhou Veterinary Research Institute
[0032] (2) Instruments and reagents: The ultracentrifuge was Sorvall WX Ultra, the rotor was Surespin 630 horizontal rotor, both were purchased from Thermo Company, the sucrose was purchased from Xilong Chemical Co., Ltd., and the ultrafiltration concentration tube was purchased from Millipore Company
[0033] 2. Experimental steps:
[0034] (1) Concentrate virus by ultrafiltration: Use an ultrafiltration concentration tube with a molecular weight cut-off of 30kDa, centrifuge at 5000g for 25min at 4°C, and concentrate 100ml of virus liquid to 2ml.
[0035] (2) Sucrose density gradient centrifugation: prepare 35ml of 15%-45% (w / v) uniform sucrose density gradient solution in a 38ml ultracentrifuge tube, take 2ml of the virus concentrate ...
Embodiment 2
[0043] Example 2 Sensitivity detection of 146S antigen detected by high performance liquid chromatography and standard curve drawing
[0044] The high performance liquid chromatography system was purchased from Agilent, and the size exclusion high performance liquid chromatography column TSK G4000 SW XL Purchased from Tosohaas Company,
[0045] (1) Dilution of pure 146S antigen: the pure 146S antigen obtained in Example 1 was diluted with 20mM phosphate buffer (pH7.0-pH7.5) by 1, 2, 4, 10, 30, 150, 250 times;
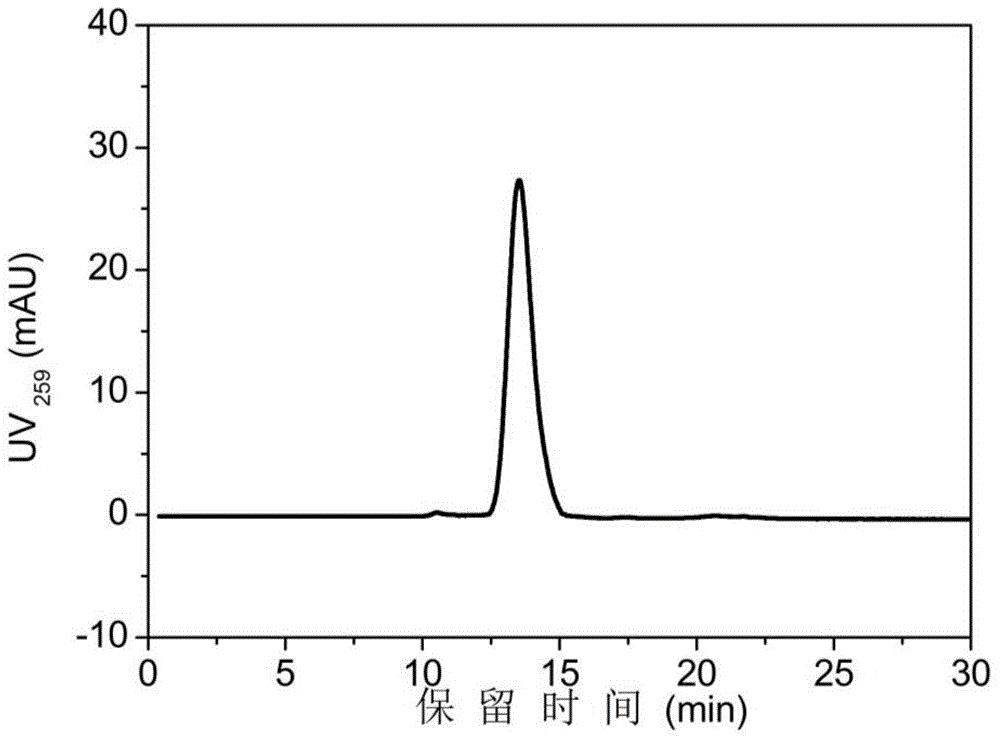
[0046] (2) Size-exclusion high-performance liquid chromatography analysis: a high-performance liquid chromatography system and a size-exclusion high-performance liquid chromatography column were used for analysis. Mobile phase: Phosphate buffer (pH7.2) containing 0.1M sodium sulfate, flow rate: 0.6ml / min, column: TSK G4000 SW XL , the injection volume is 100μl, and the detection wavelength is 259nm.
[0047] (3) Establish a standard curve: after HPLC gel filtration ...
Embodiment 3
[0050] Example 3 Determination of 146S antigen in different serotypes of foot-and-mouth disease vaccine finished products.
[0051] With the method of the invention, the content of 146S antigen in the finished products of foot-and-mouth disease vaccines of different serotypes can be determined. The following vaccines were used as samples for determination: a. O-type and Asian type I bivalent inactivated vaccine (ONXC strain + JSL strain) b. O-type inactivated porcine foot-and-mouth disease vaccine (O / Mya98 / xj / 2010 strain + O / GX / 09-7 strain) c. swine foot-and-mouth disease type O inactivated vaccine (O / GX / 09-7 strain+O / XJ / 10-11 strain).
[0052] (1) Pretreatment of finished vaccine: Since adjuvants are added to the finished vaccine, demulsification is required before measurement. Add n-butanol according to 1 / 9 of the volume of the vaccine stock solution, mix well, let stand at 4°C for 1 hour, centrifuge at 5000rpm for 2 minutes, and collect the bottom solution containing the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| sedimentation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com