Application of tiacumicin derivative to preparation of medicine for treating related diseases and/or symptoms caused by dengue virus infection
A technology of taigamycin and dengue virus, applied in the field of medicine, can solve the problem of lack of effective drugs, etc., and achieve the effects of high safety, strong binding ability, and high activity of inhibiting dengue virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Anti-DENV activity of tiacumicin derivatives at the cellular level
[0022] Virus strain: Dengue virus DENV2 (NGC)
[0023] Cell line: A549
[0024] Detection method:
[0025] Tiacumicin derivative antiviral half effective dose (50% Effective Concentration, EC 50 ): DMSO, gradient concentrations of tiacumicin derivatives to saturate the cells 1 h in advance, after 2 h of virus infection, replace the virus-free medium containing the corresponding concentration of drugs for 48 h; collect the cell supernatant, extract the viral RNA in the supernatant, and use qRT- PCR method was used to detect the copy number of DENV2 virus RNA in the supernatant.
[0026] Inhibition rate (%) = (1-viral RNA copy number of drug administration group / viral RNA copy number of solvent control group) 100%, calculated using the Forcast formula of EXCEL 2013, when the inhibition rate is equal to 50%, corresponding to tiacumicin derivative The concentration of the substance, as EC 5...
Embodiment 2
[0031] Example 2 The inhibitory activity of tiacumicycin derivatives to DENV2-prM protein in A549 cell line
[0032] Virus strain: Dengue virus DENV2 (NGC)
[0033] Cell line: A549
[0034] Detection method:
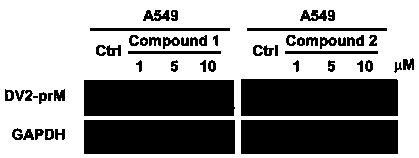
[0035] DMSO, 1, 5, 10 μM tiacumicin derivatives were used to saturate the cells 1 hour in advance, and 2 hours after the virus infection, the virus-free medium containing the corresponding concentration of drugs was replaced for 48 hours; the cell pellet was collected, and the cells under different treatments were detected by western blot electrophoresis The relative expression of internal DENV2 (NGC)-prM protein, with GAPDH as internal reference protein. Such as figure 1 . Tiacumicin derivatives Compound 1 and Compound 2 can effectively inhibit the expression of DENV2prM protein at a concentration of 1-5 μM. As an important structural protein, the expression of DENV2prM protein is inhibited, which means that the reproduction of DENV2 has been inhibited.
Embodiment 3
[0036] Example 3 Surface plasmon resonance detection of affinity between tiacumicin derivatives and DENV2-NS5 protein
[0037] This method adopts GE's Biacore T100 instrument and CM5 chip for experiments. First, the purified DENV2 (NGC)-NS5 protein is amino-coupled to the CM5 chip, and then flows through different concentrations of tiacumicin derivatives, and the instrument detects The mass change of the substance adsorbed on the surface of the chip is calculated to calculate the affinity rate of the compound (K a ) vs. dissociation rate (K d ). Affinity = dissociation equilibrium constant (K D ), this value describes the binding strength between a small molecule and a protein molecule. The biological meaning is that when a small molecule binds to a protein 1:1, let 50% of the small molecule saturate the concentration of the protein molecule. The smaller the value, the stronger the binding. Usually, the affinity between small molecules and proteins calculated by this method...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com