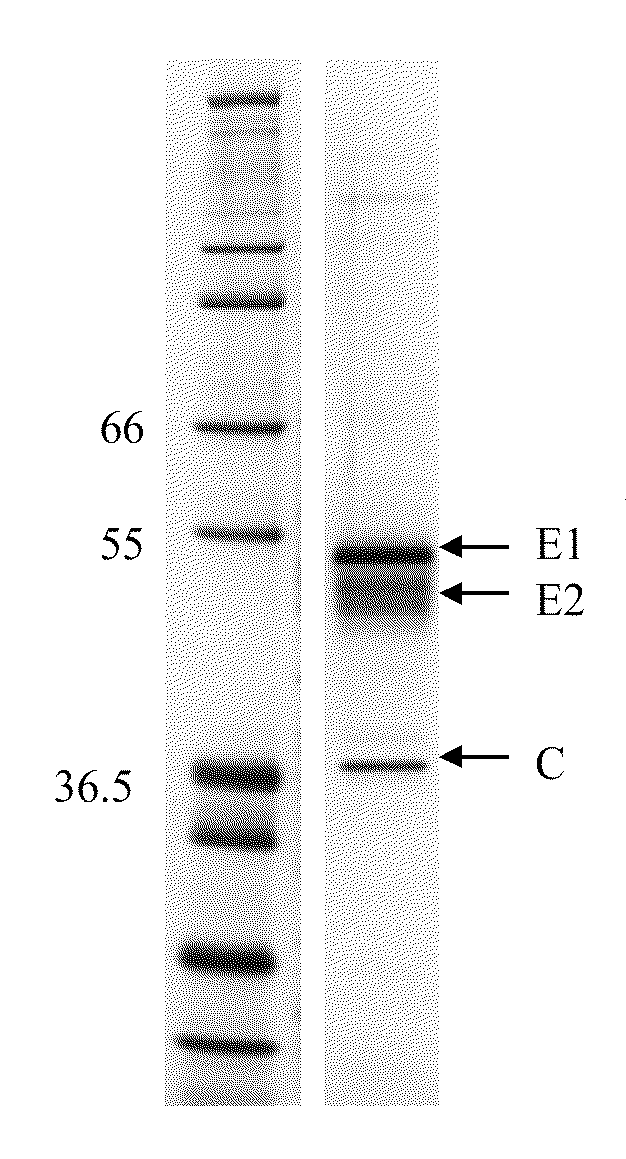
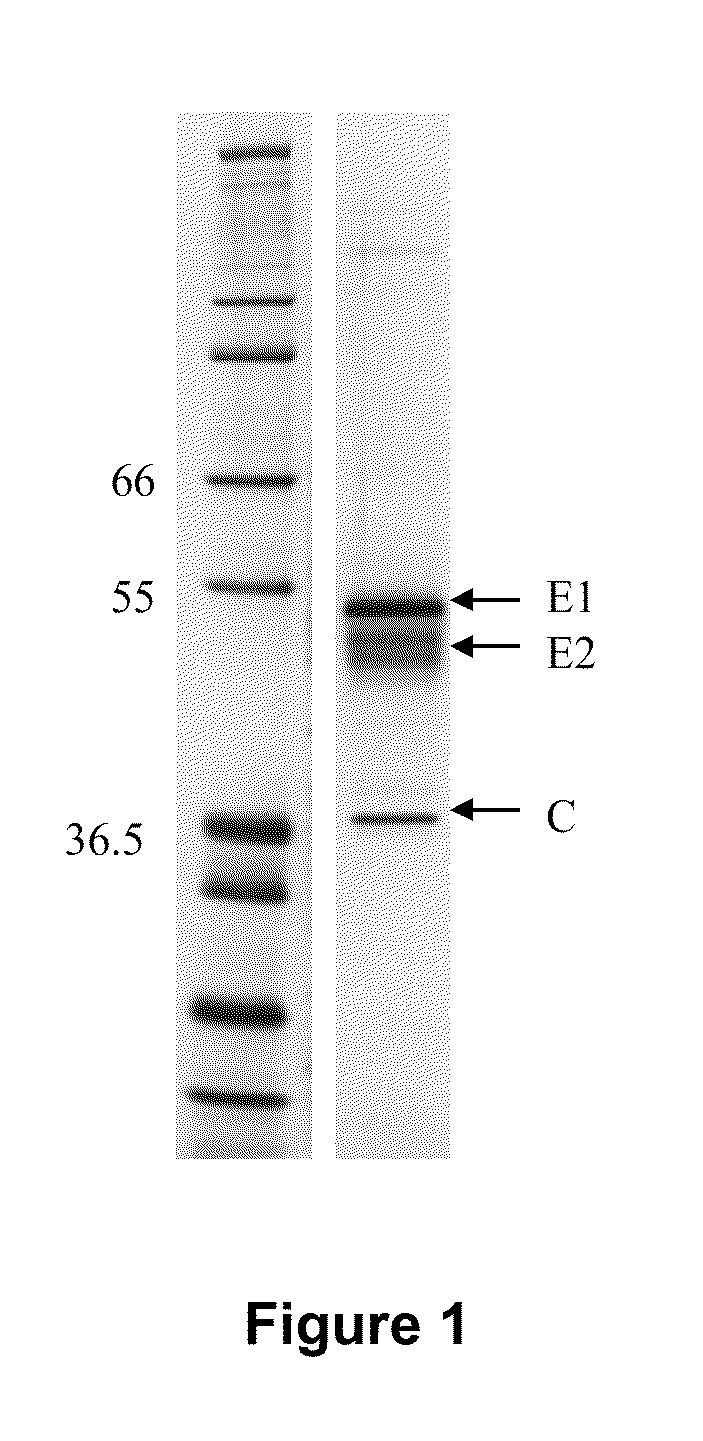
Alphavirus and Alphavirus Replicon Particle Formulations and Methods
a technology of alphavirus and replicon particle, applied in the field of alphavirus and alphavirus replicon particle formulations and methods, can solve the problems of high cost of system, inability to deliver vaccines, and inability to heat up vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0085]Various types of excipients were evaluated to assess their ability to stabilize GFP VRP during freezing, drying, storage at 37° C., and rehydration.
Reagents
[0086]Sucrose, dextran (average MW 40 kDa), Tween 80™, and mannitol were obtained from Spectrum Chemical, Gardena, Calif. Sodium sulfate was purchased from JT Baker, Phillipsburg, N.J. Sodium citrate, HES (6% solution in 0.9% NaCl), and pH 7.4 preset Tris crystals were obtained from Sigma (St. Louis, Mo.). A 25% solution of HSA (Buminate™, Baxter Healthcare Corp. Westlake Village, Calif.) and Water for Irrigation (WFI) were obtained from Baxter. Where available, all of these reagents were at minimum NF grade.
[0087]For the genome quantitation assay, molecular biology grade water was obtained from Eppendorf. 1× Tris-EDTA (TE) buffer, pH 8.0, and a 10% SDS solution were purchased from Ambion. The Universal RT Master Mix was obtained from Applied Biosystems, Foster City, Calif., and the NSP2 primers and probe were purchased fro...
example 2
[0111]An ARP based on Venezuelan equine encephalitis virus, i.e. a VRP, expressing the heavy chain of botulinum neurotoxin type B (BoNT / B Hc) in the replicon RNA was lyophilized in the presence of sodium phosphate, sucrose, HSA, and sodium chloride (buffer, sugar, surfactant, salt). FIG. 15 plots infectivity data during storage under frozen and refrigerated conditions (−20° C. and 2-8° C., respectively). Under frozen conditions, infectivity decreased very slightly (<0.2 log) after almost two years. Under refrigerated conditions, the infectivity dropped less than 1 log after almost two years.
example 3
[0112]An ARP based on Venezuelan equine encephalitis virus, i.e. a VRP, expressing green fluorescent protein (GFP) in the replicon RNA was lyophilized in the presence of sodium phosphate, sucrose, HSA, sodium sulfate, methionine (1 mM), and glycerol (buffer, sugar, surfactant, salt, antioxidant, plasticizer). FIG. 16 plots infectivity data during storage under frozen and refrigerated conditions (−20° C. and 2-8° C., respectively). Under both frozen and refrigerated conditions, infectivity remained essentially unchanged after six months, followed by a decrease of about 0.5 log when stability was assessed over twelve months. While methionine may be present, it is believed not to be required for stability.
[0113]FIGS. 16B-16C plot infectivity data when this same matrix is used to formulate an ARP based on Venezuelan equine encephalitis virus, i.e. a VRP, expressing influenza virus hemagglutinin (HA) surface protein (A / WY H3) in the replicon RNA from two separate production processes. In...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com