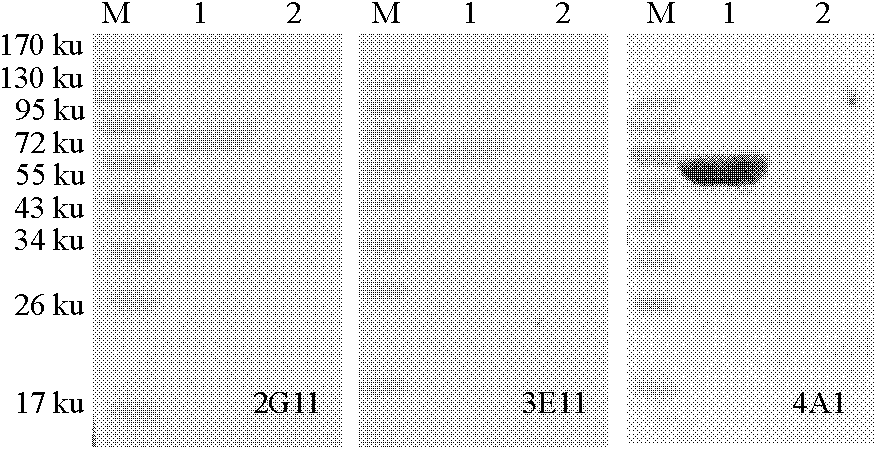Patents
Literature
34 results about "Equine herpesvirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Equine herpesvirus is a group of viruses of the family Herpesviridae that affect horses. Equine herpesvirus 1 of the subfamily Alphaherpesvirinae and genus Varicellovirus Equine herpesvirus 2 of the subfamily Gammaherpesvirinae and genus Rhadinovirus Equine herpesvirus 3 of the subfamily Alphaherpesvirinae and genus Varicellovirus Equine herpesvirus 4 of the subfamily Alphaherpesvirinae and genus Varicellovirus Equine herpesvirus 5 of the subfamily Gammaherpesvirinae and genus Rhadinovirus
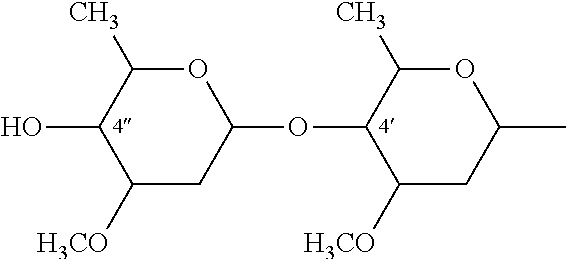
Equine herpesvirus glycoproteins
InactiveUS6193983B1Improve identityPeptide/protein ingredientsGenetic material ingredientsHerpes simplex virus DNAEquine herpesvirus
A vaccine for the selective immunization of horses against EHV4 and / or EHV1 is provided comprising at least one of (i) EHV4 virus wherein a portion of the gG gene of the EHV4 virus that elicits a type-specific response to EHV4 has been deleted and (ii) EHV1 virus wherein a portion of the gG gene of the EHV1 virus that elicits a type-specific response to EHV1 has been deleted. Antibodies which specifically bind to a epitopes of EHV4 gG or EHV1 also are provided.
Owner:UNIVERSITY OF MELBOURNE
Equine herpesvirus glycoproteins
InactiveUS6544526B1Peptide/protein ingredientsViral antigen ingredientsHerpes simplex virus DNAEquine herpesvirus
A vaccine for the selective immunization of horses against EHV4 and / or EHV1 is provided comprising at least one of (i) EHV4 virus wherein a portion of the gG gene of the EHV4 virus that elicits a type-specific response to EHV4 has been deleted and (ii) EHV1 virus wherein a portion of the gG gene of the EHV1 virus that elicits a type-specific response to EHV1 has been deleted. Antibodies which specifically bind to a epitopes of EHV4 gG or EHV1 also are provided.
Owner:UNIVERSITY OF MELBOURNE
Application of polycyclic polyketides in preparation of anti-HV (herpes virus) drug
The invention discloses an application of polycyclic polyketides in preparation of an anti-HV (herpes virus) drug. It is found that the polyketides can inhibit diseases caused by infection of four HVs including HSV-1 (herpes simplex virus-1), HSV-2 (herpes simplex virus-2), VZV (varicella zoster virus) and CMV (cytomegalo virus). The compounds show equivalent activity but have different acting mechanisms as compared with commercial drugs such as acyclovir and can overcome drug resistance of existing commercial drugs. Therefore, the compounds have good application prospects in treatment of related diseases caused by infection of HVs including HSV-1, HSV-2, VZV and CMV.
Owner:JINAN UNIVERSITY
Attenuated equine herpesvirus
InactiveUS7060282B1Reduced virulenceViral antigen ingredientsVirus peptidesVirulent characteristicsViral Genes
The present invention relates to novel Equine herpesvirus (EHV) mutants comprising one or more deletions, substitutions, or insertions in the endogenous promoter region of an essential viral gene, preferably the immediate early gene of EHV. The EHV mutants are stable and have reduced virulence, which makes them very suitable for use in a live vaccine. The invention furthermore relates to live vaccines comprising said EHV mutants, to DNA sequences and vectors harbouring a mutated EHV sequence, to host cells transfected with said DNA or vectors. The invention also relates to a method of attenuating EHV in general, and EHV-1 in particular.
Owner:INTERVET INT BV
Recombinant equine herpesvirus-1 vaccine containing mutated glycoprotein c and uses thereof
The present invention provides compositions or vaccines that contain a recombinant EHV-1 that elicit an immune response in animals against equine herpesvirus, including compositions comprising said recombinant EHV-1, methods of vaccination against equine herpesvirus, and kits for use with such methods and compositions.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
New swine influenza vaccine
ActiveUS20180080044A1Low costSimilar efficiencySsRNA viruses negative-senseViral antigen ingredientsNucleotideHerpes simplex virus DNA
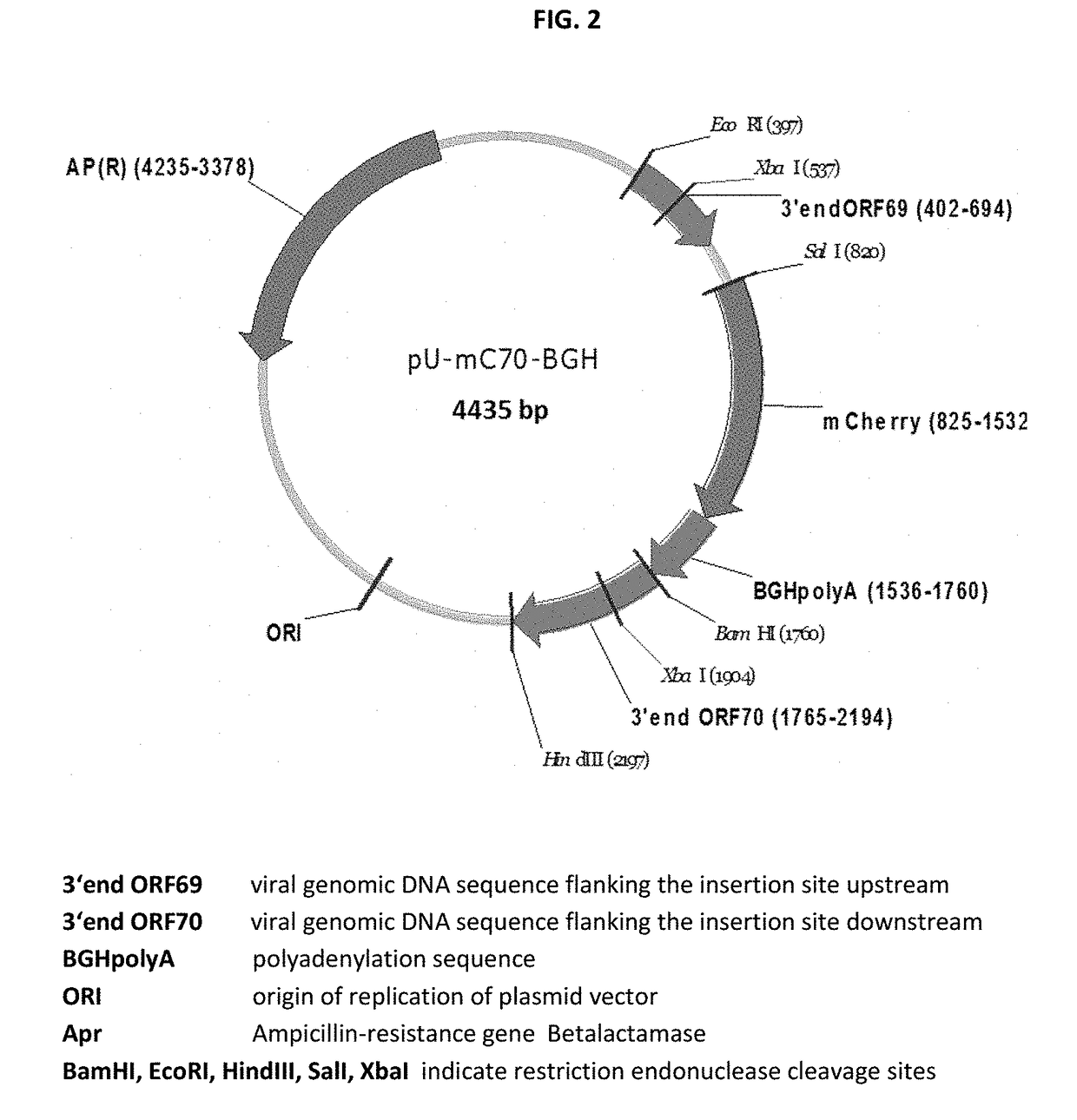
The present invention relates to Equine Herpes Virus (EHV) vectors comprising at least one exogenous antigen encoding sequence relating to a pathogen infecting food producing animals, wherein said exogenous antigen encoding sequence is inserted into an insertion site, preferably ORF70, and said exogenous antigen encoding sequence is operably linked to a promoter sequence, preferably the promoter sequence comprising 4pgG600 (SEQ ID NO:1) or 4pMCP600 (SEQ ID NO:2) or the complementary nucleotide sequences thereof or a functional fragment or a functional derivative thereof or the complementary nucleotide sequences thereof. Furthermore, the present invention relates to methods for immunizing a food producing animal comprising administering to such food producing animal an immunogenic composition comprising embodiments of the present invention. Moreover, the present invention relates to methods for the treatment or prophylaxis of clinical signs caused by swine influenza virus in a food producing animal.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Recombinant equine herpesvirus-1 vaccine containing mutated glycoprotein C and uses thereof
The present invention provides compositions or vaccines that contain a recombinant EHV-1 that elicit an immune response in animals against equine herpesvirus, including compositions comprising said recombinant EHV-1, methods of vaccination against equine herpesvirus, and kits for use with such methods and compositions.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Application of polycyclic polyketides in preparation of anti-herpesvirus drugs
The invention discloses application of polycyclic polyketides in the preparation of anti-herpesvirus drugs. According to the invention, the polyketides are found to inhibit diseases caused by herpes simplex virus (HSV) type I, HSV-2, varicella zoster virus (VZV) and cytomegalo virus (CMV) infection. Compared with drugs such as acyclovir on the market, the compound in the invention exhibits considerable activity, but the mechanism of action is different, and the compound overcomes the drug resistance of the drugs currently on the market. Thus, these compounds have good application prospects inthe treatment of diseases caused by HSV-1, HSV-2, VZV and CMV infection.
Owner:JINAN UNIVERSITY
Lamp detection primers and Lamp detection method for discrimination of equine herpesvirus type 1/4
InactiveCN103757131AStrong specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesNoseDiagnosis methods
The invention discloses Lamp (loop-mediated isothermal amplification) detection primers and a Lamp detection method for discrimination of equine herpesvirus type 1 / 4 and application thereof, belonging to the field of molecular biological diagnosis methods for animal virosis. A corresponding detection primer group is designed according to the gB gene sequence of equine herpesvirus by using Lamp technology and comprises outer primers FIP and BIP and inner primers F3 and B3, and the Lamp detection method for typing equine herpesvirus type 1 and equine herpesvirus type 4 is invented with the detection primer group as the detection primers. According to the invention, equine herpesvirus infection and typing and other functions at the level of DNA can be realized by carrying out extraction of total DNA and Lamp amplification on equine nose swab, equine brain tissue and lung tissue samples, and the characteristics of easiness, convenience, fastness, good specificity, easy determination of results and the like are obtained.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
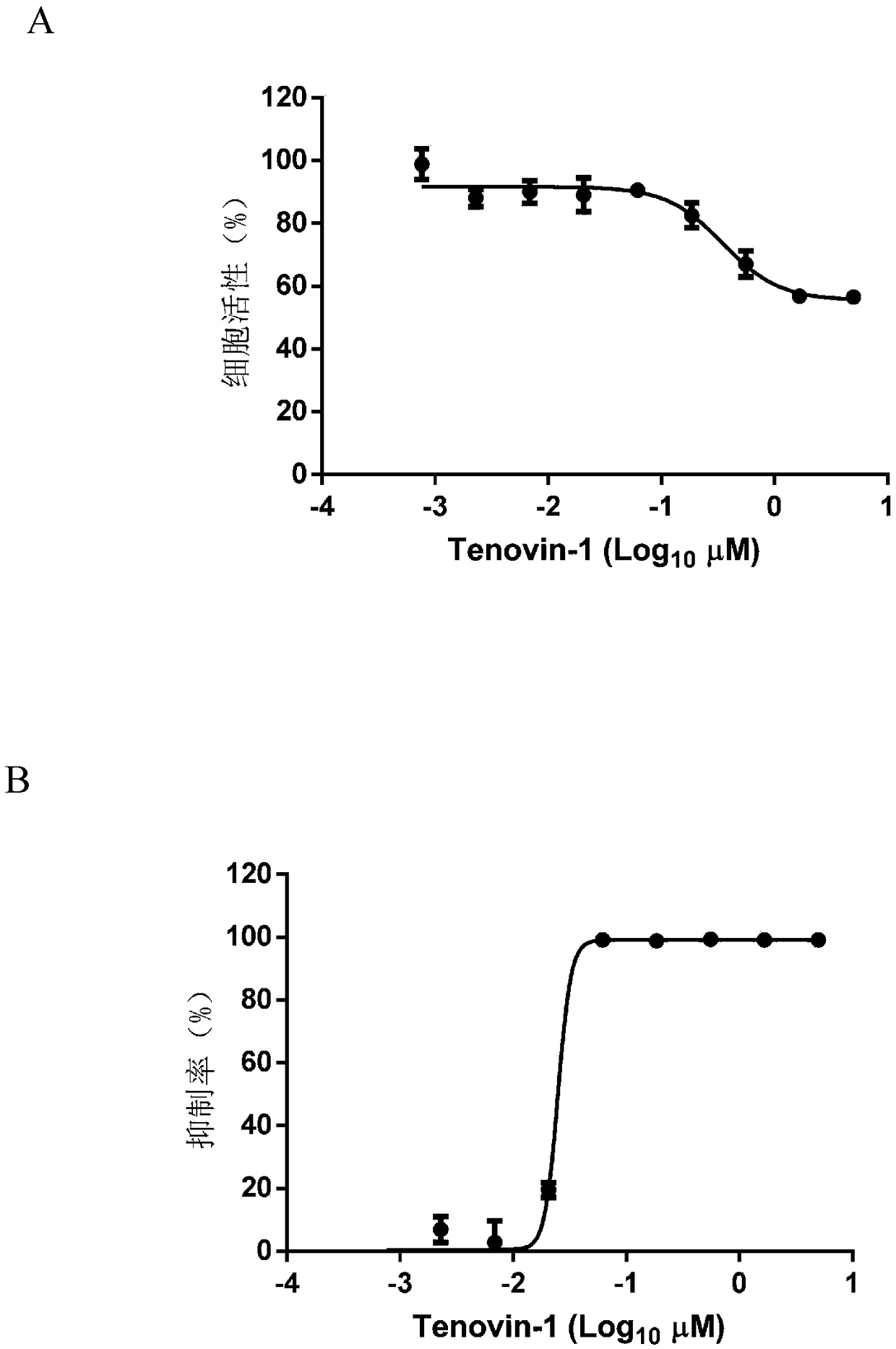
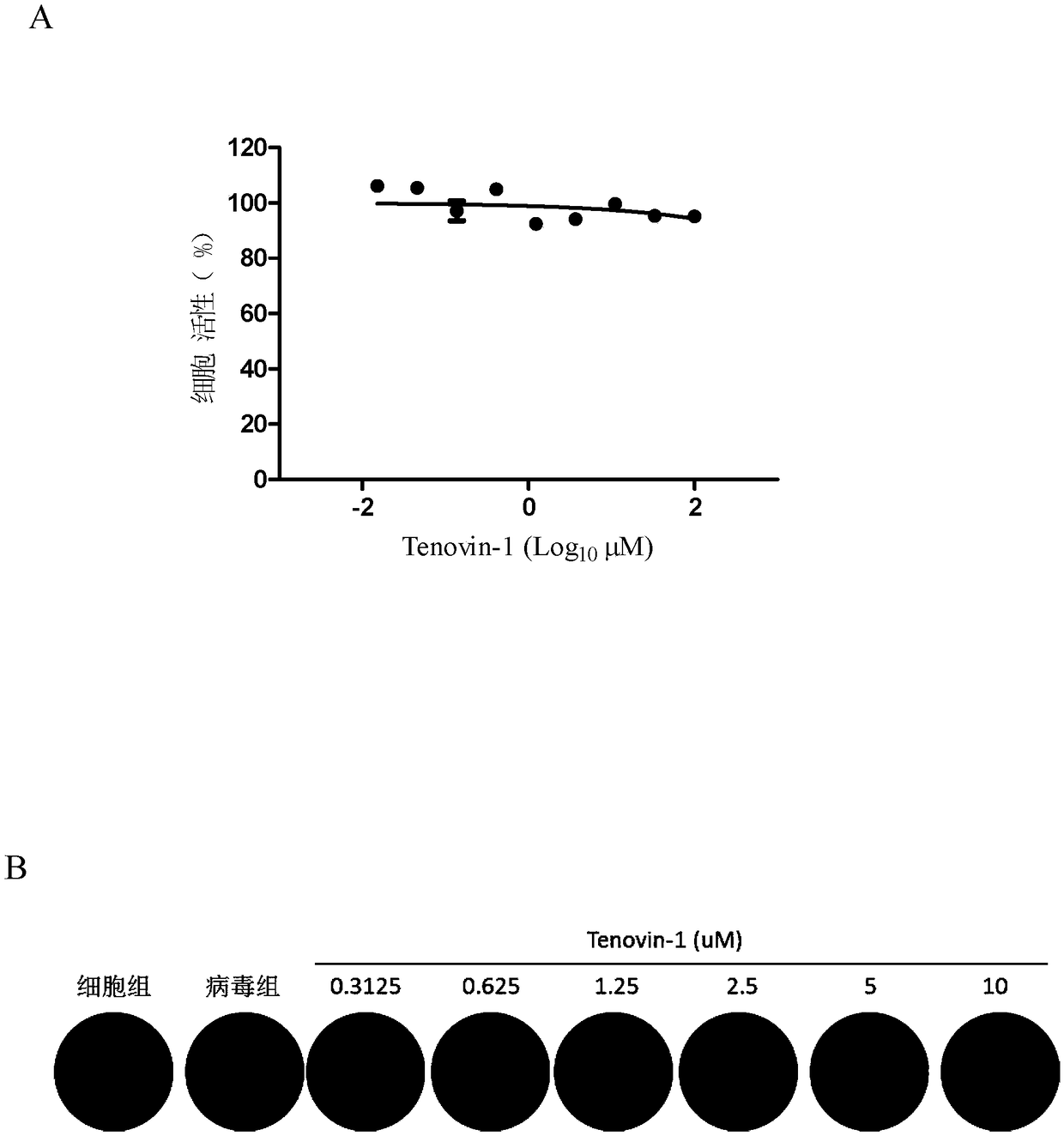
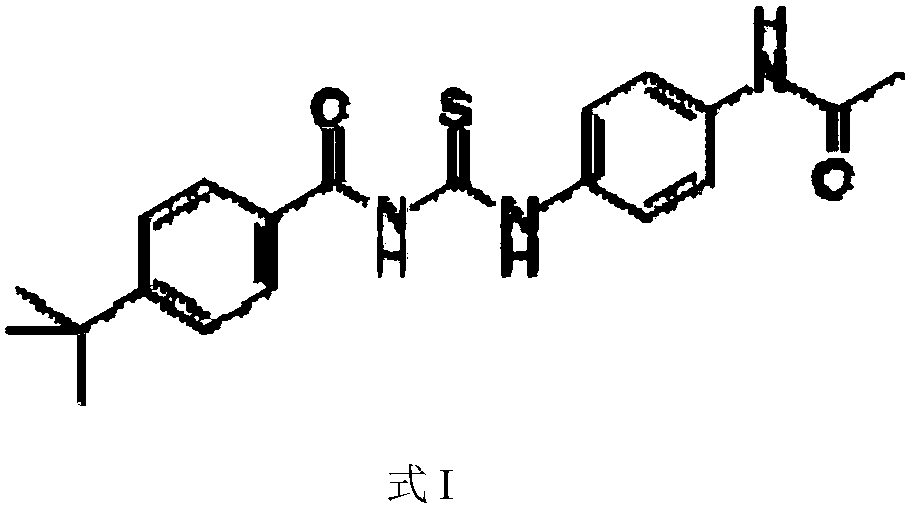
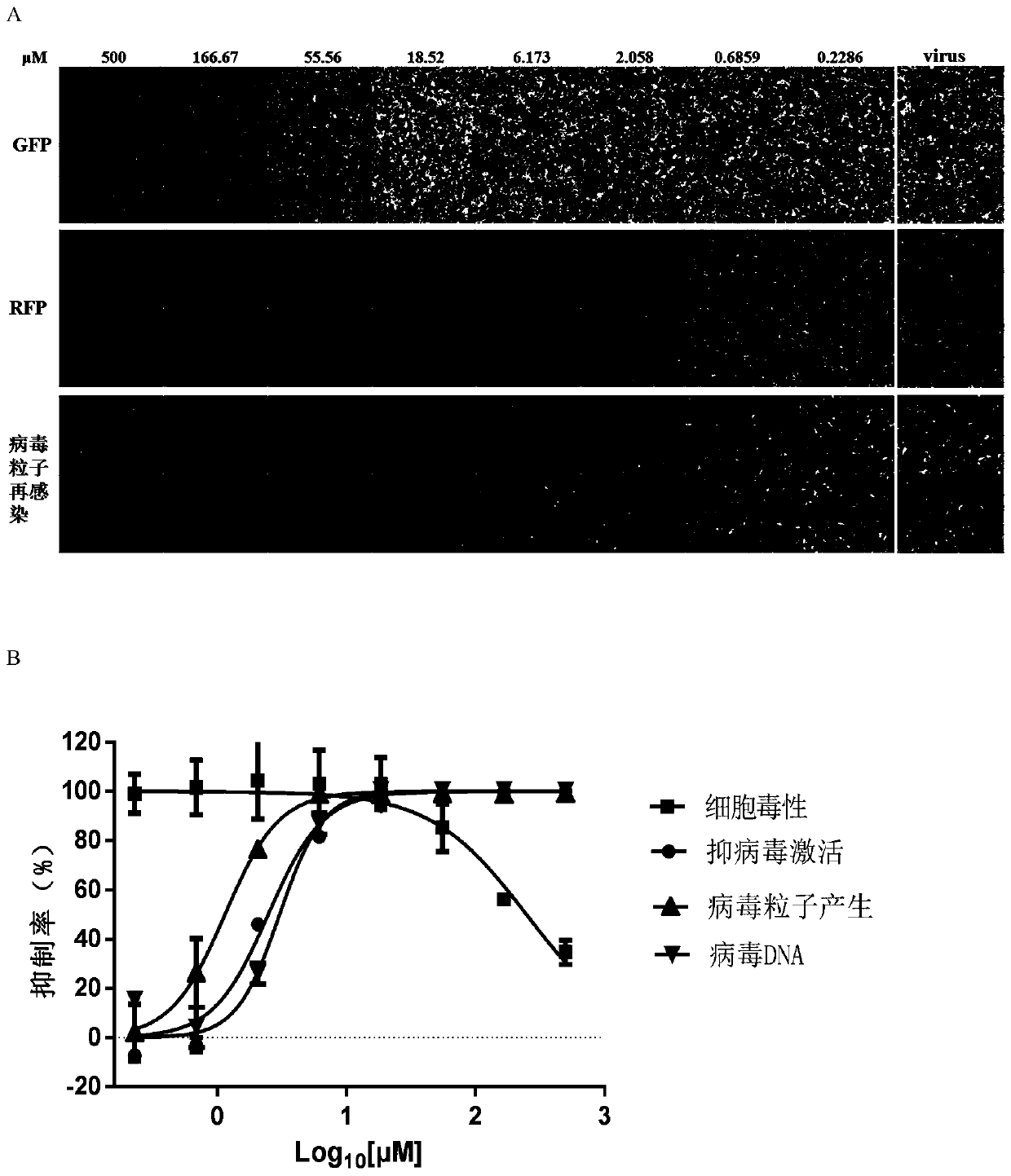
Application of Tenovin-1 in preparing drug for preventing and controlling human herpes virus infection
InactiveCN108619123AAvoid infectionGood inhibitory effectAntiviralsAmide active ingredientsHerpes simplex virus DNAInfection disease
The invention provides a use of Tenovin-1 in preparing a drug. The drug is used for treating or preventing herpes virus infection. According to the embodiment of the invention, the Tenovin-1 can be effectively used for treating or preventing herpes virus infection. Detection on herpes viruses in different subtypes shows that, the Tenovin-1 has the activity of broad-spectrum anti-herpes viruses, ishigh in selection index, can be developed into a drug for treating herpes virus infection diseases and has a wide application prospect.
Owner:武汉威立得生物医药有限公司
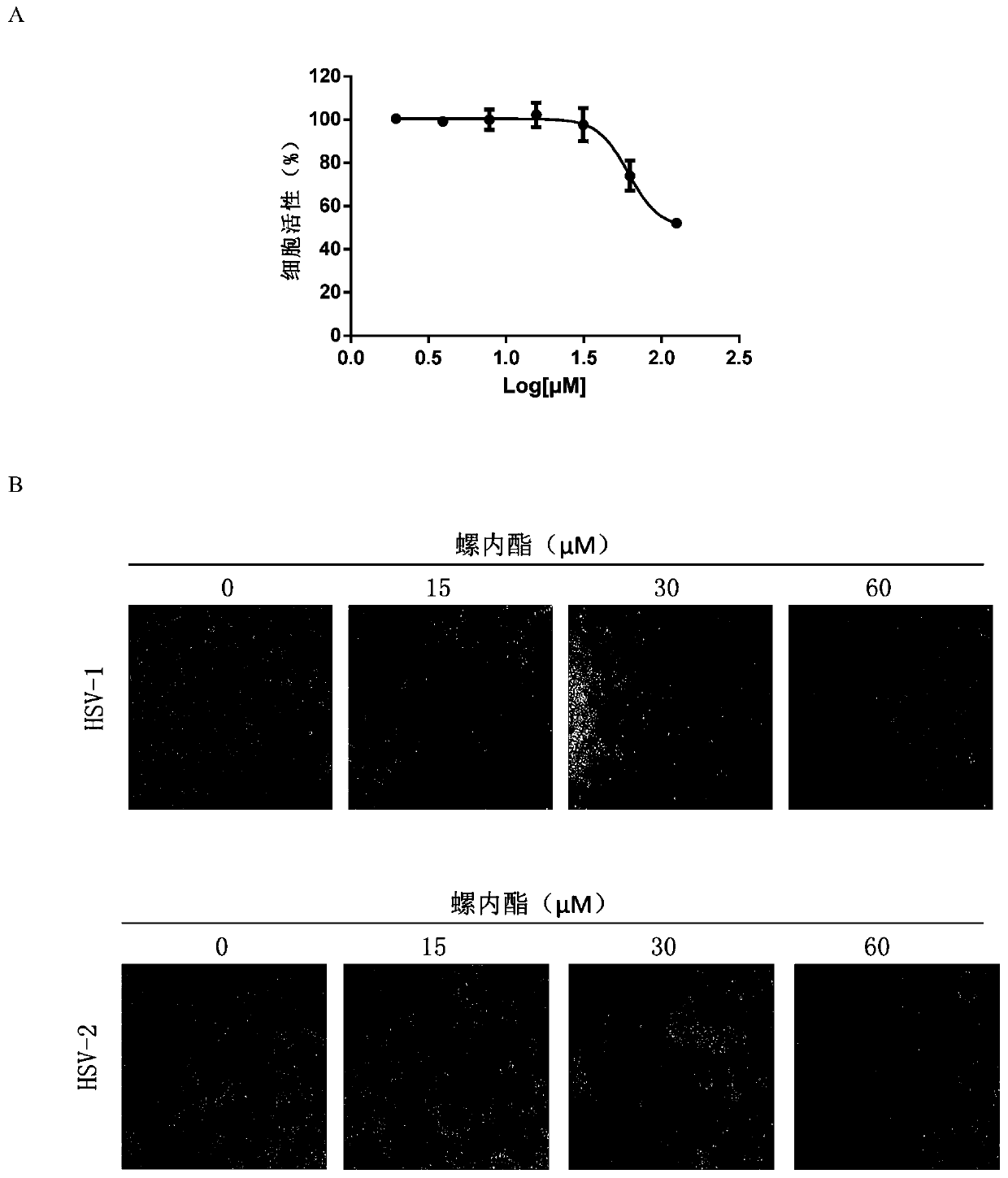
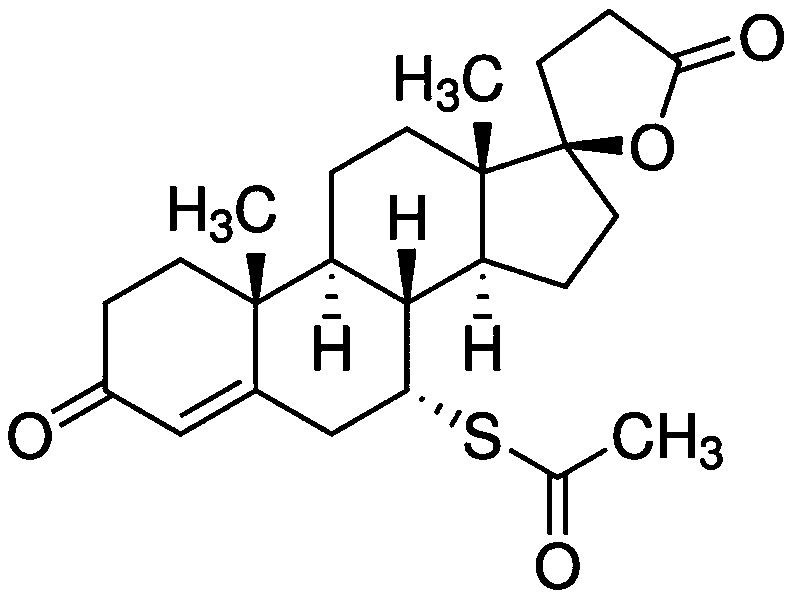
Application of spironolactone in preparation of drug for treating human herpesvirus infection
InactiveCN109925317AActive Broad SpectrumHigh activityOrganic active ingredientsAntiviralsSide effectCytopathic effect
The invention discloses application of spironolactone in preparation of a drug for treating human herpesvirus infection. We evaluate the effect of resisting Kaposi's sarcoma-associated herpes virus KSHV and herpes simplex viruses HSV-1 and HSV-2 of the spironolactone by measuring the level of infectious virion production, the level of DNA replication during lysis, or the degree of cytopathic effect caused by the virus. The spironolactone has significant antiviral activity and a dose-dependent effect, can inhibit the activation of replication during KSHV lysis with a half inhibitory concentration (IC50) of 1.145 microM to KSHV infectious virions. At non-toxic concentration, the spironolactone is effective in reducing cytopathic effect caused by HSV-1 and HSV-2. The spironolactone is a complete inhibitor of mineralocorticoids (such as aldosterone). The spironolactone is clinically used for inhibiting sodium reabsorption, potassium excretion, diuresis and the like of organisms and has thesmall toxic and side effects on the human body. The compound can inhibit replication of herpes virus in the lysis phase, and has a wide prospect of being developed into the drug for treating human herpes virus infection.
Owner:武汉威立得生物医药有限公司
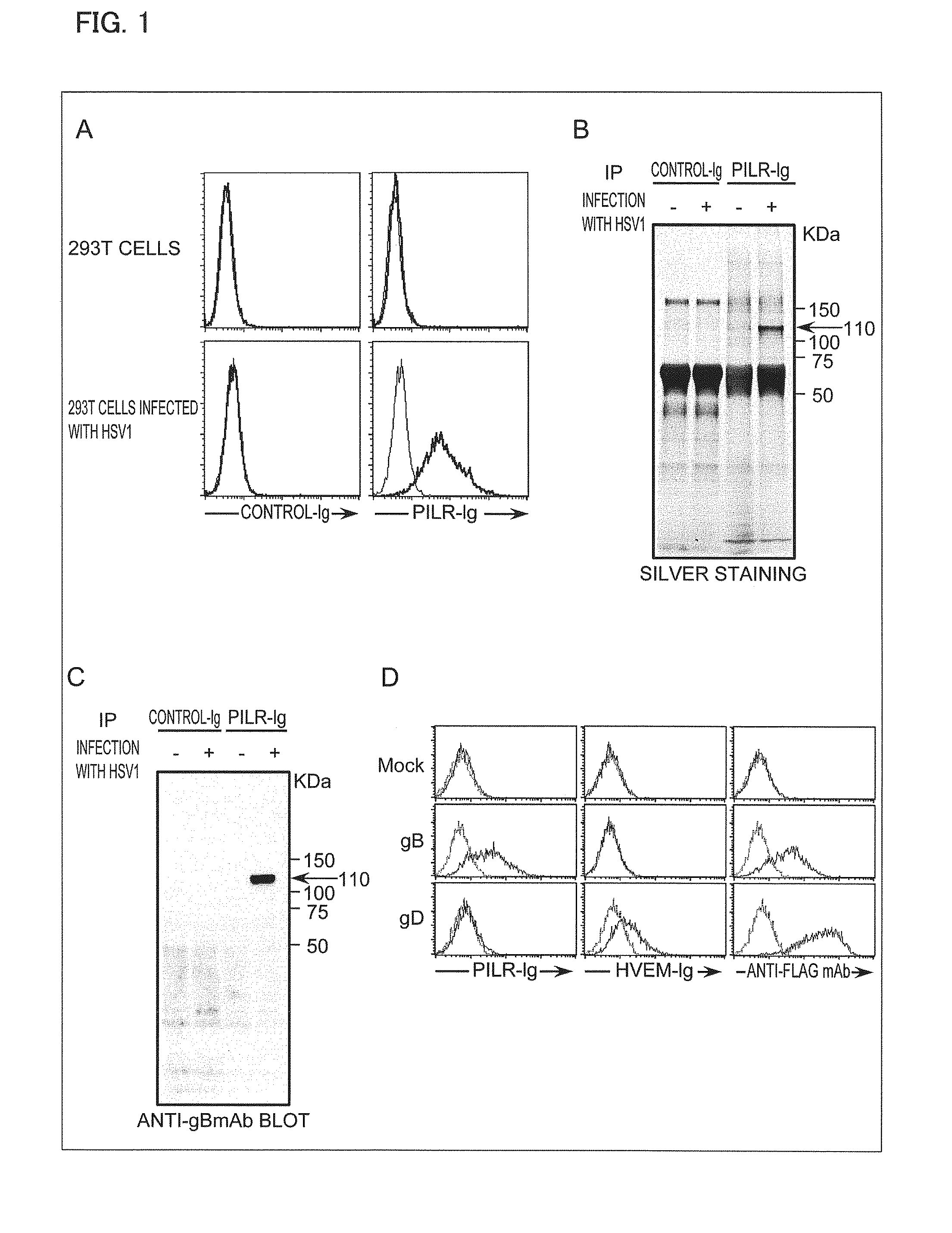
Pharmaceutical Composition for Treatment and Prevention of Herpes Virus Infections
InactiveUS20130115589A1Avoid infectionAvoid reinfectionOrganic active ingredientsPeptide/protein ingredientsHeavy chainHerpes simplex virus DNA
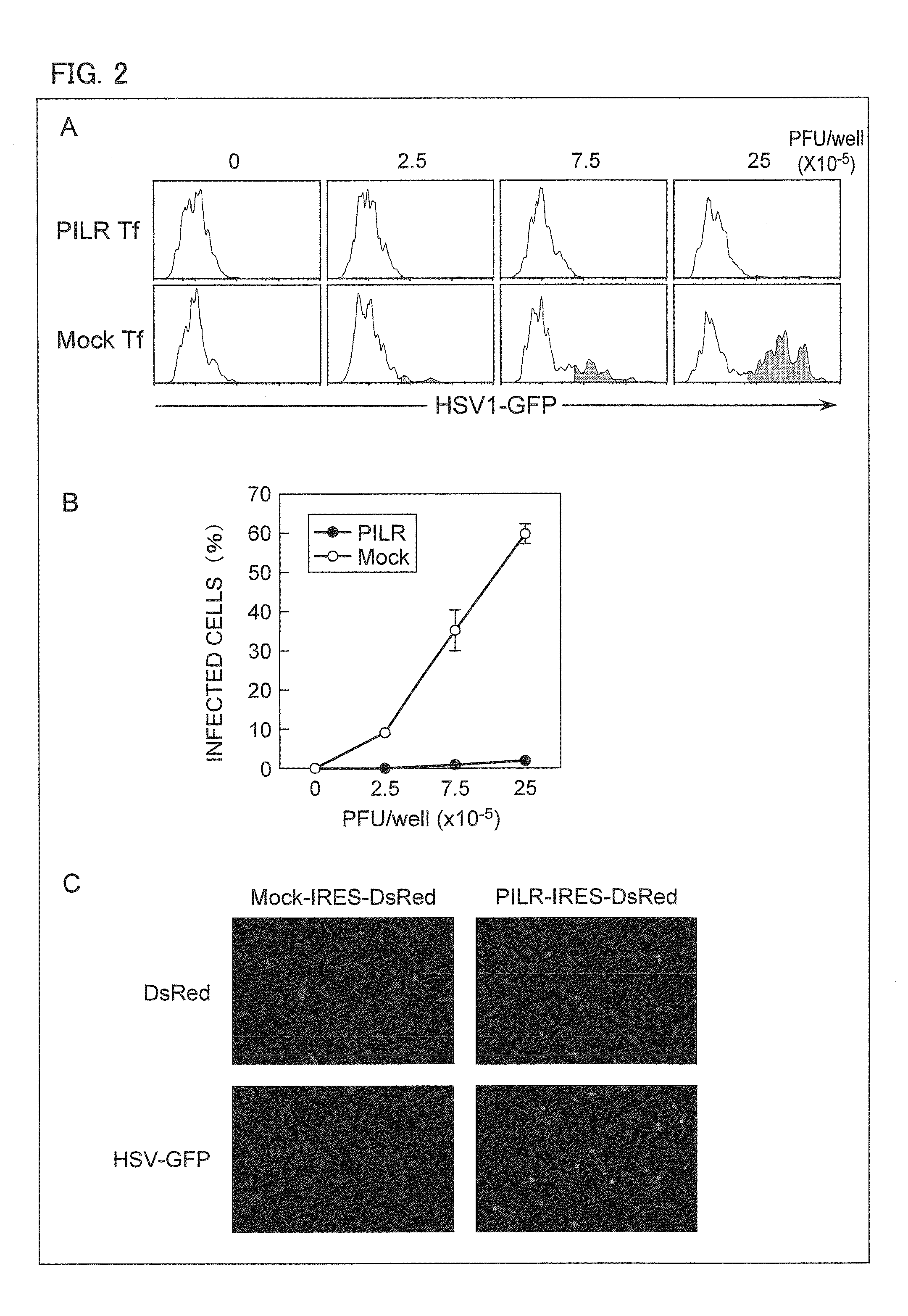
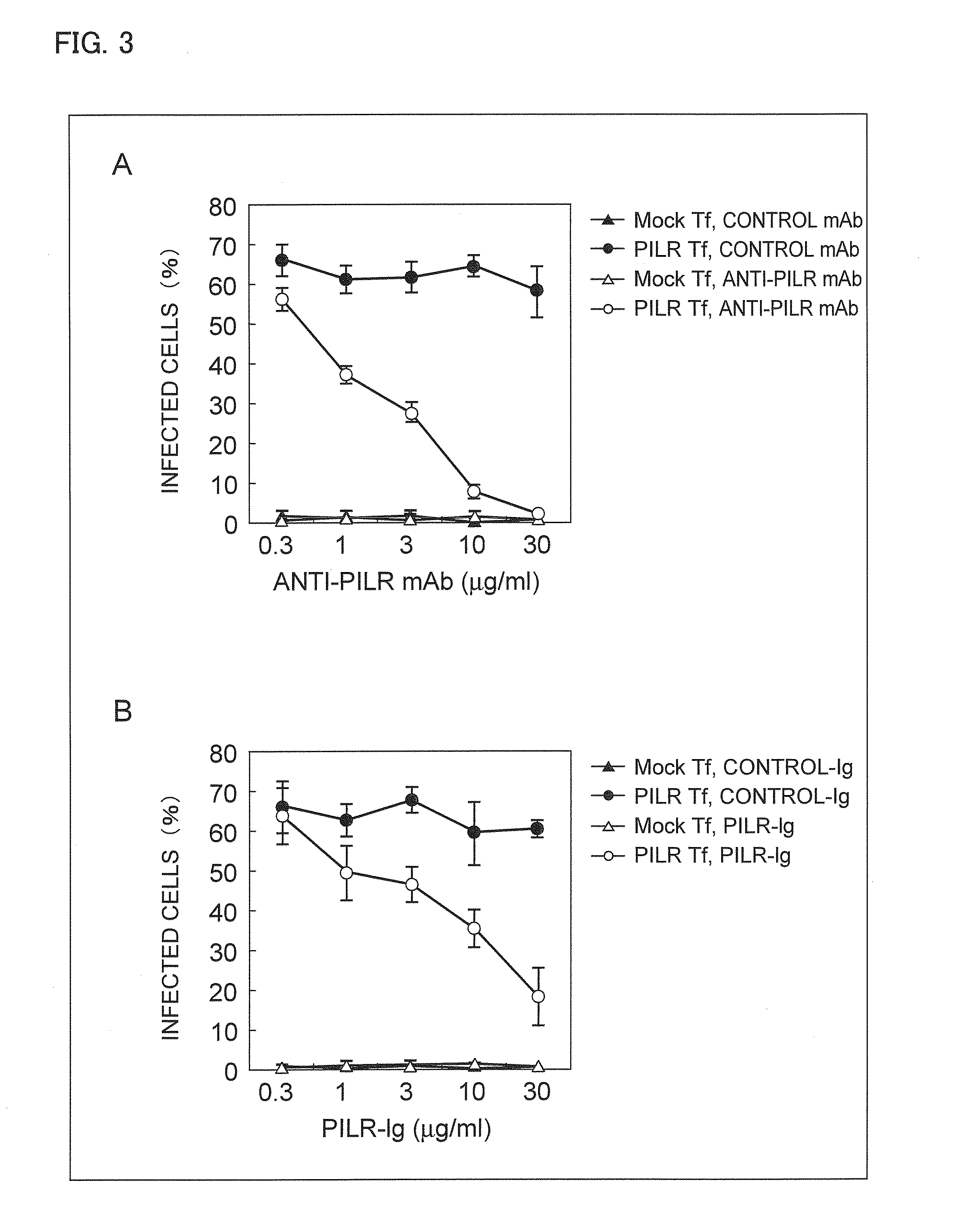
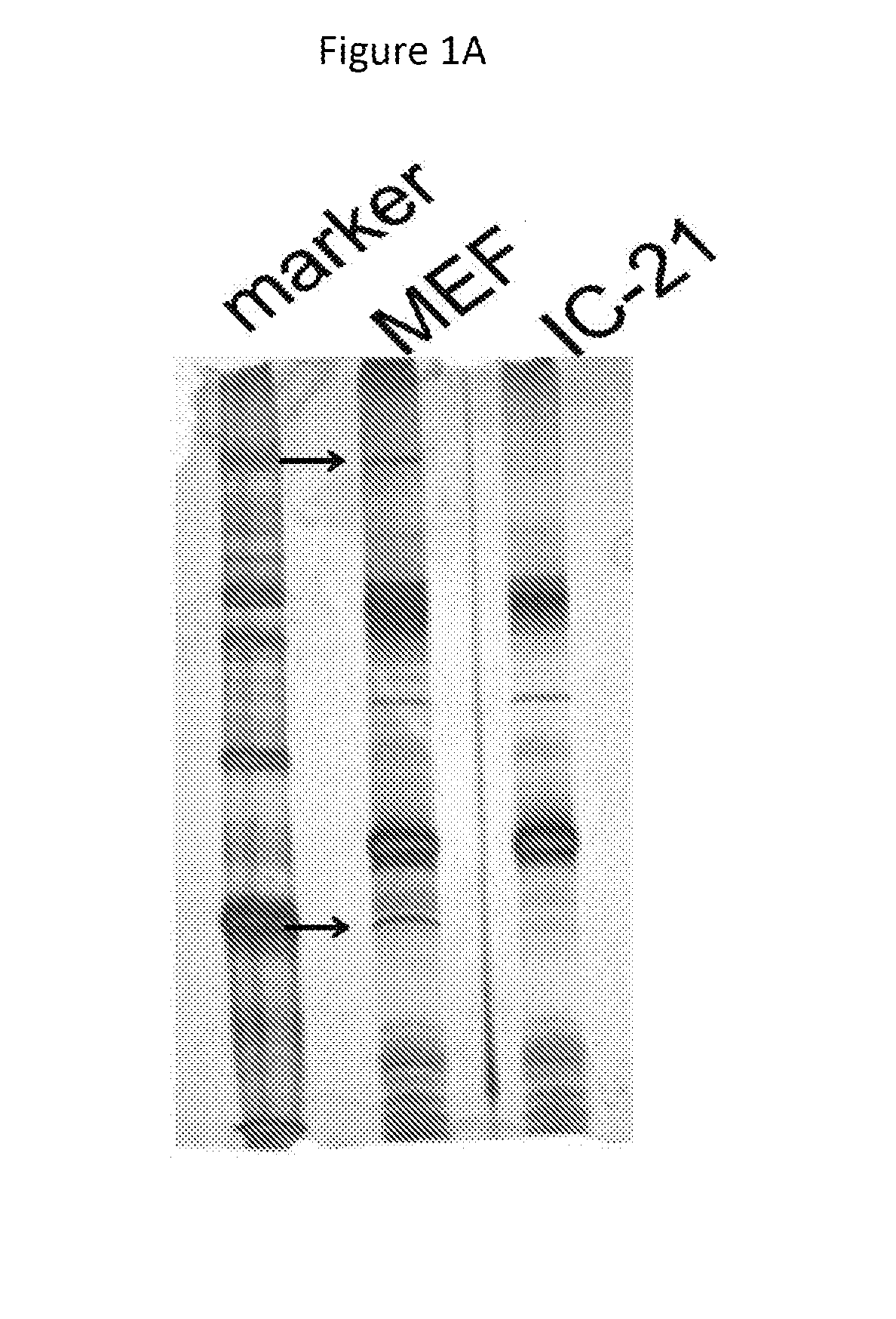
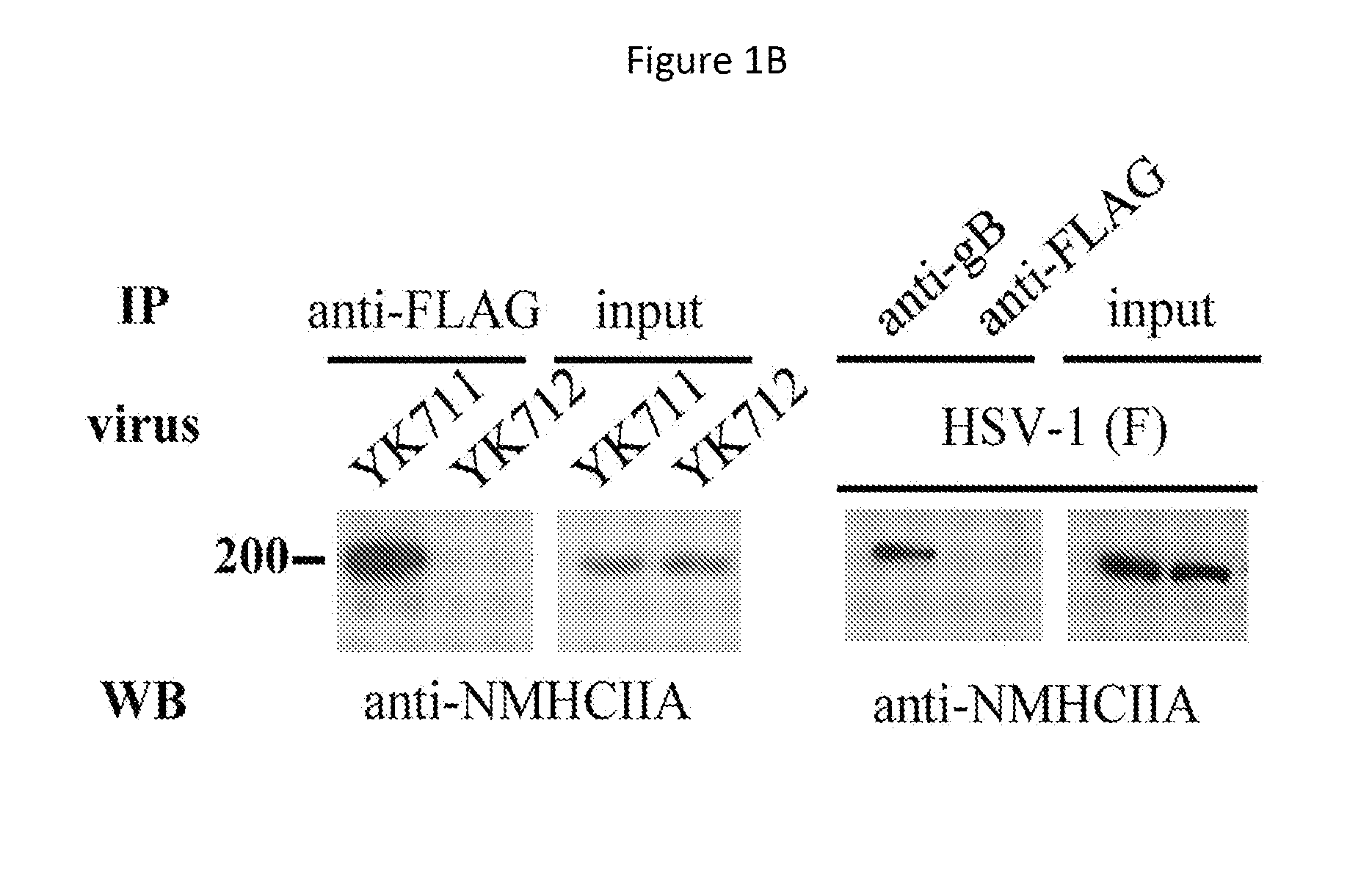
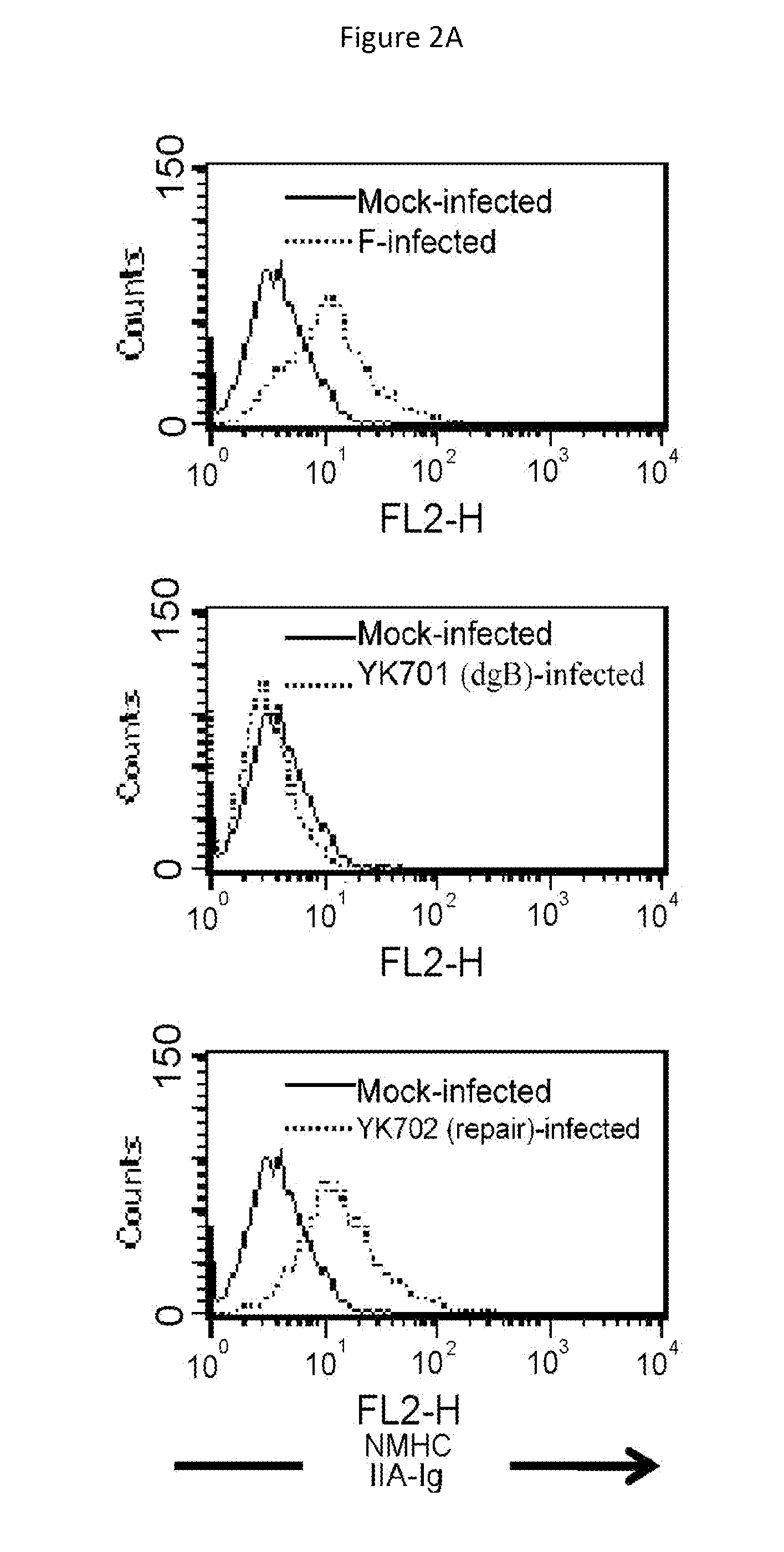
An object of the present invention is to find a protein expressed in a variety of cells and functioning as a receptor for herpesvirus and provide a preventive or remedy for herpesvirus infections capable of inhibiting binding of the receptor to herpesvirus and thereby preventing entry of the virus to cells.The present invention provides a pharmaceutical composition for preventing or treating herpesvirus infections, which composition contains a substance inhibiting the binding of glycoprotein B to a non-muscle myosin heavy chain IIA or a non-muscle myosin heavy chain.
Owner:THE UNIV OF TOKYO
Equine disease model for herpesvirus neurologic disease and uses thereof
InactiveUS20170281751A1Increase chanceProtection levelAntibacterial agentsViral antigen ingredientsNervous systemEquine herpesvirus
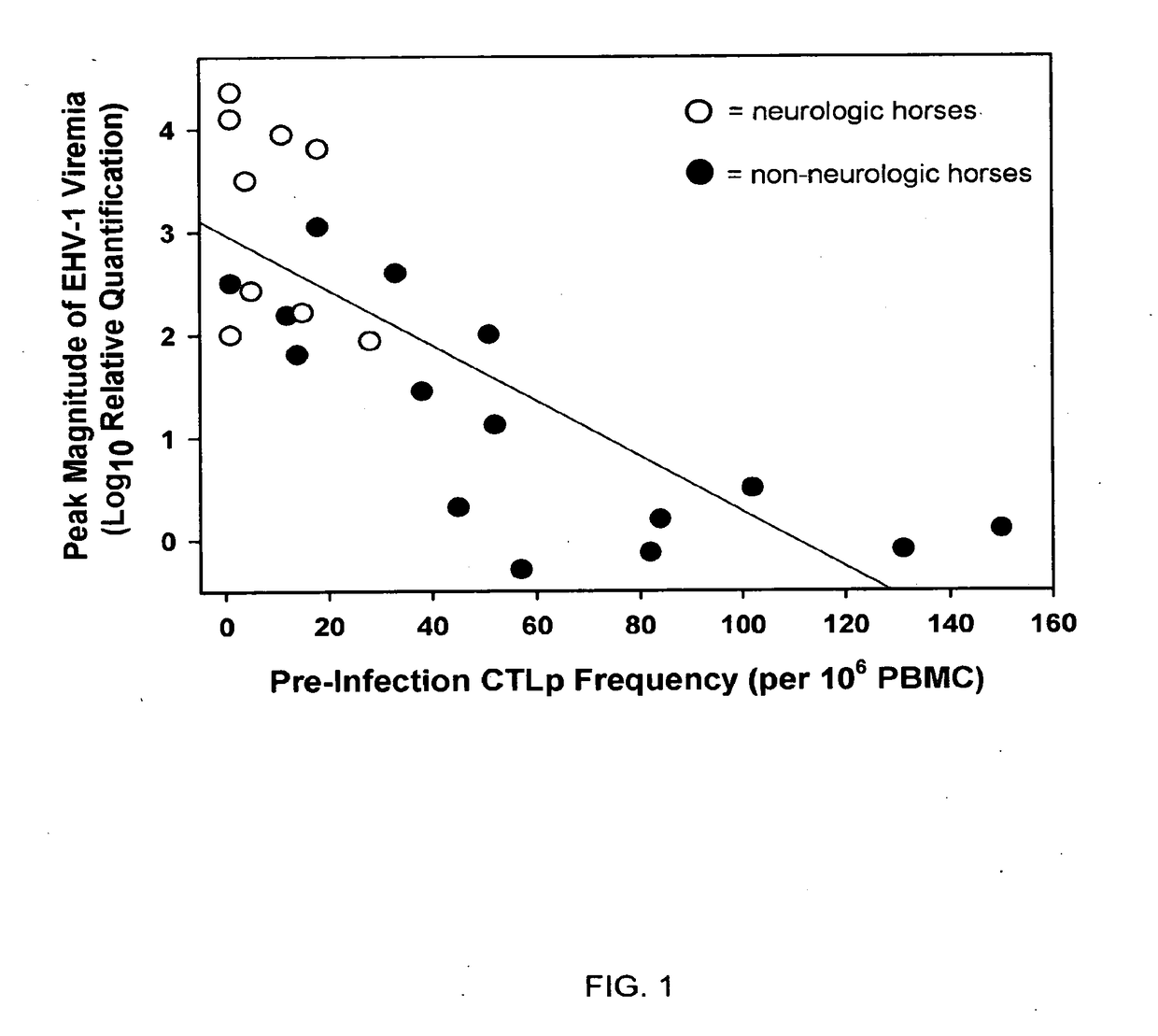
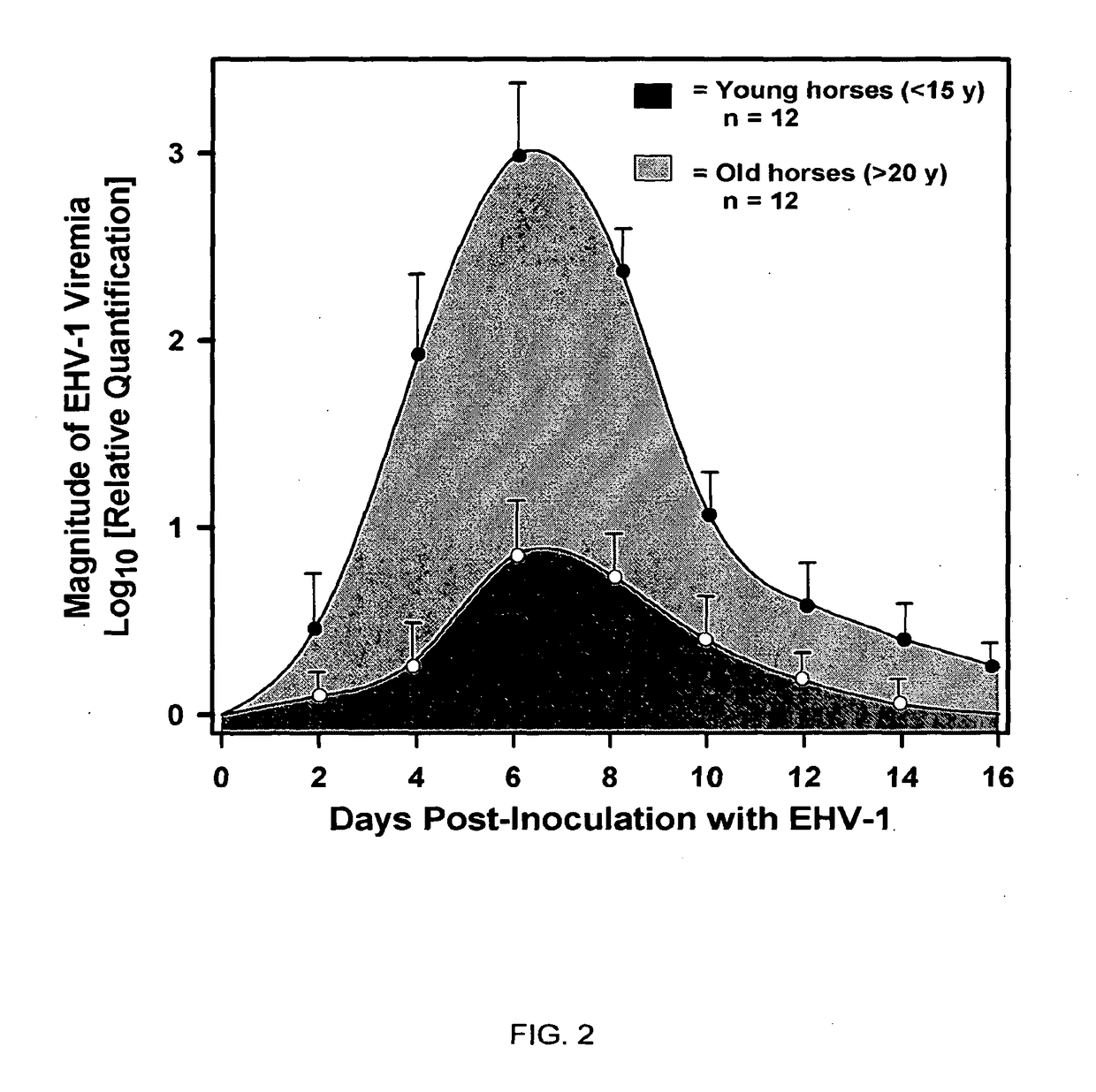
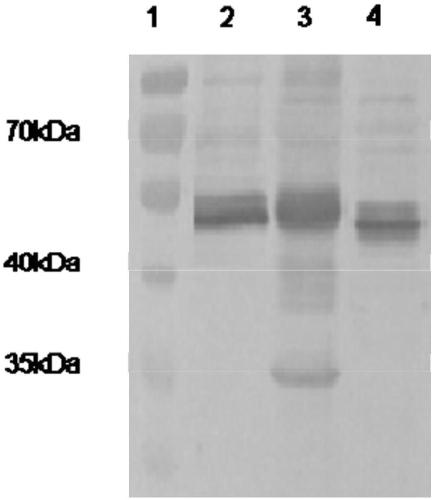
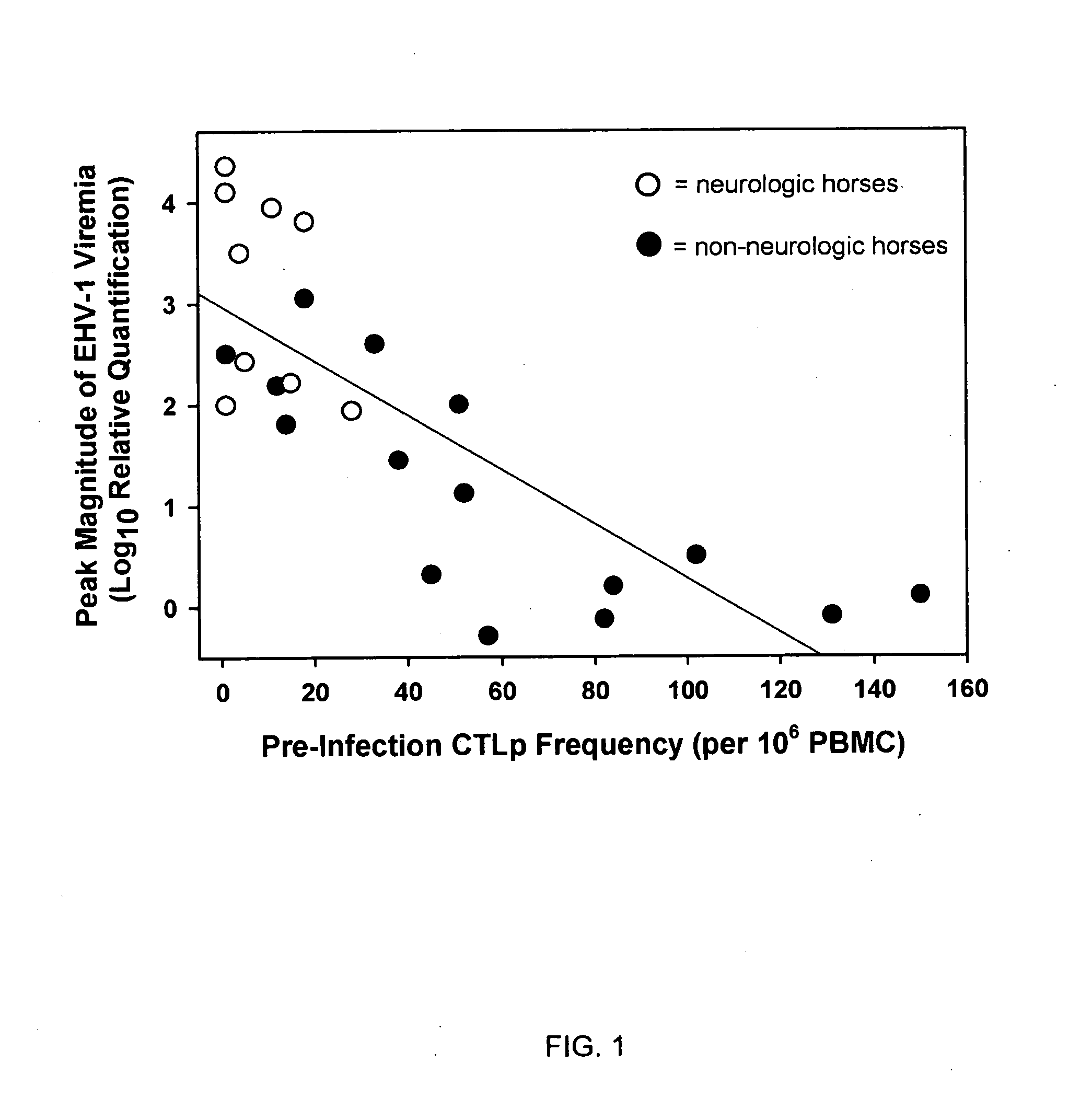
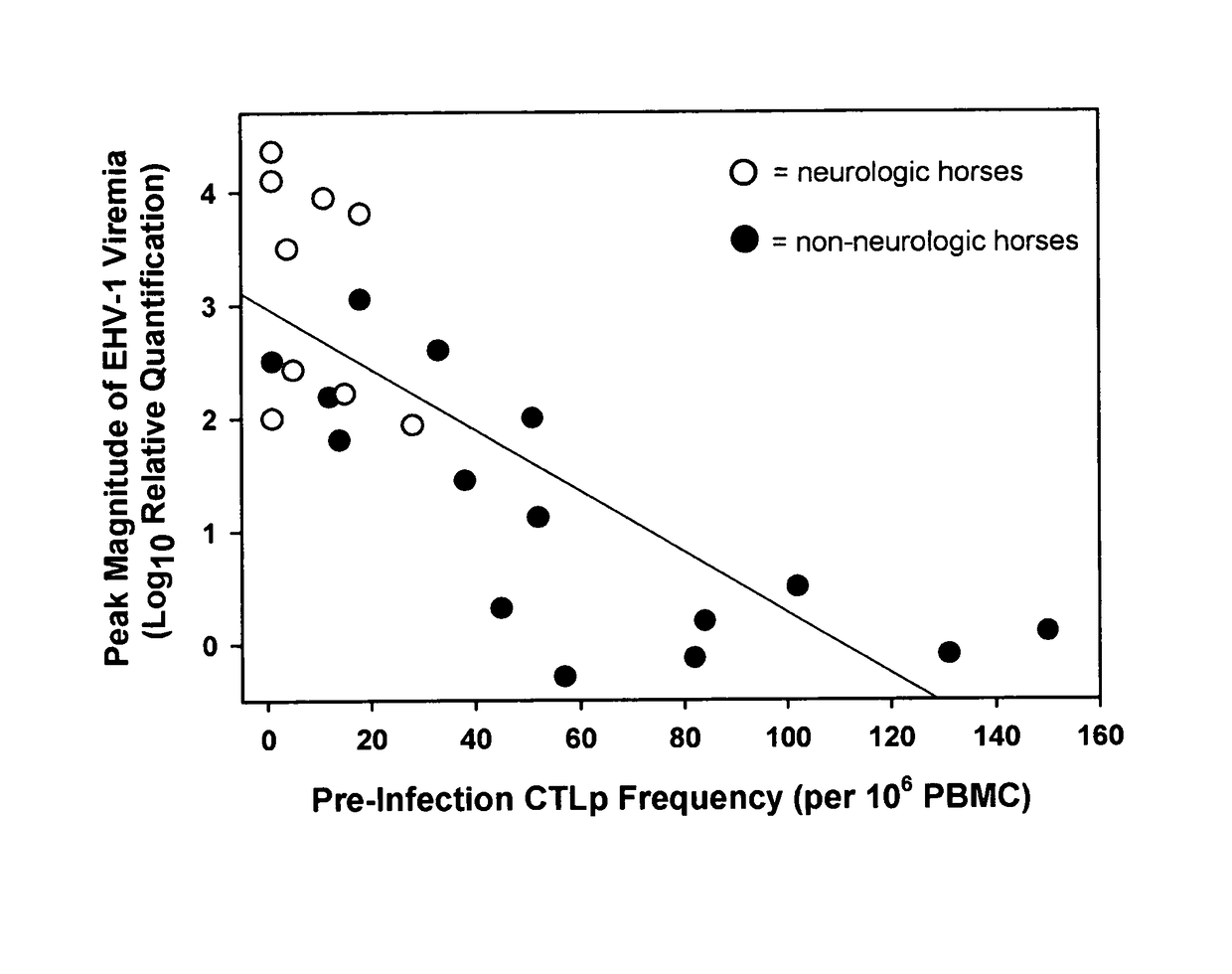
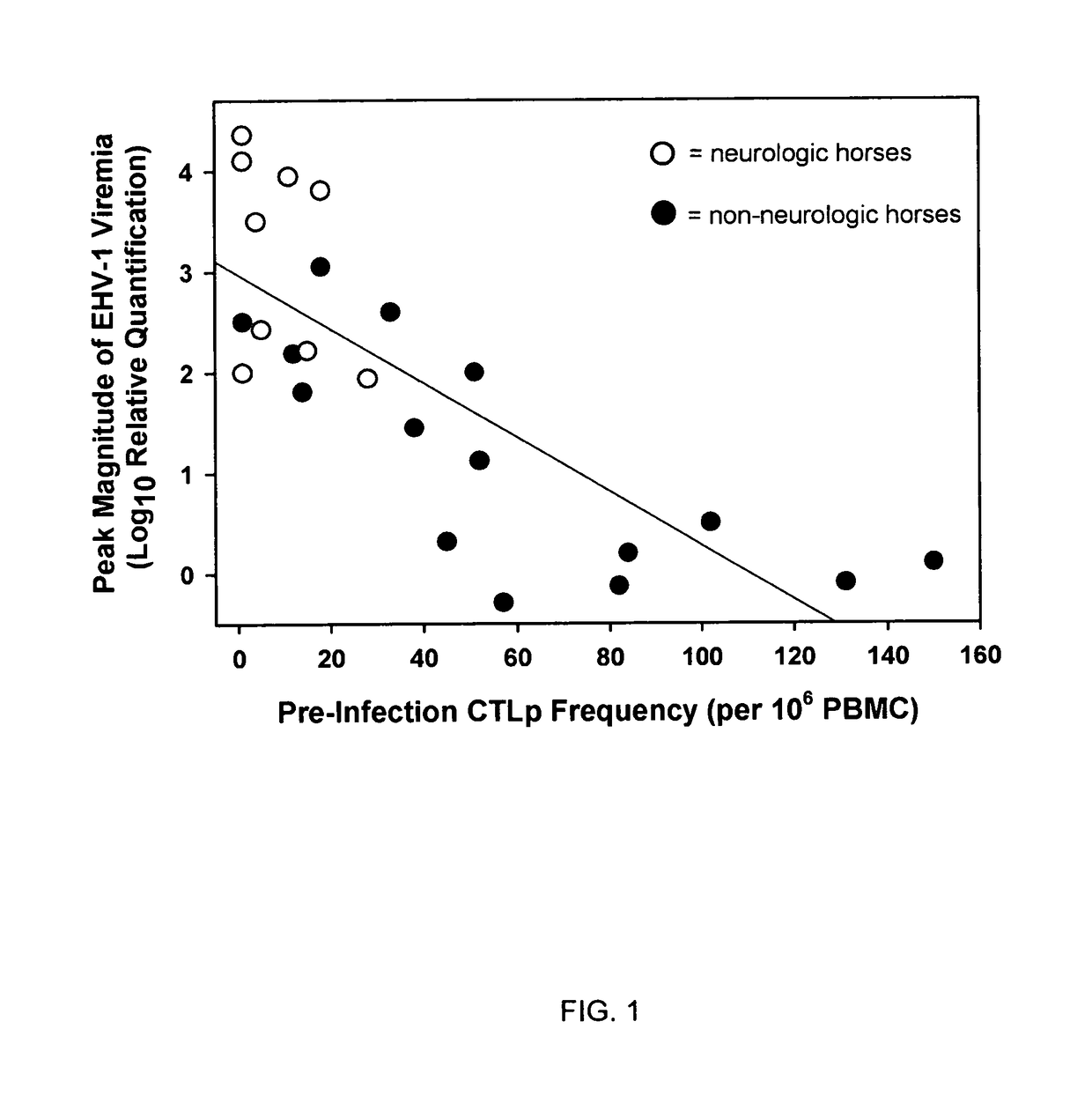
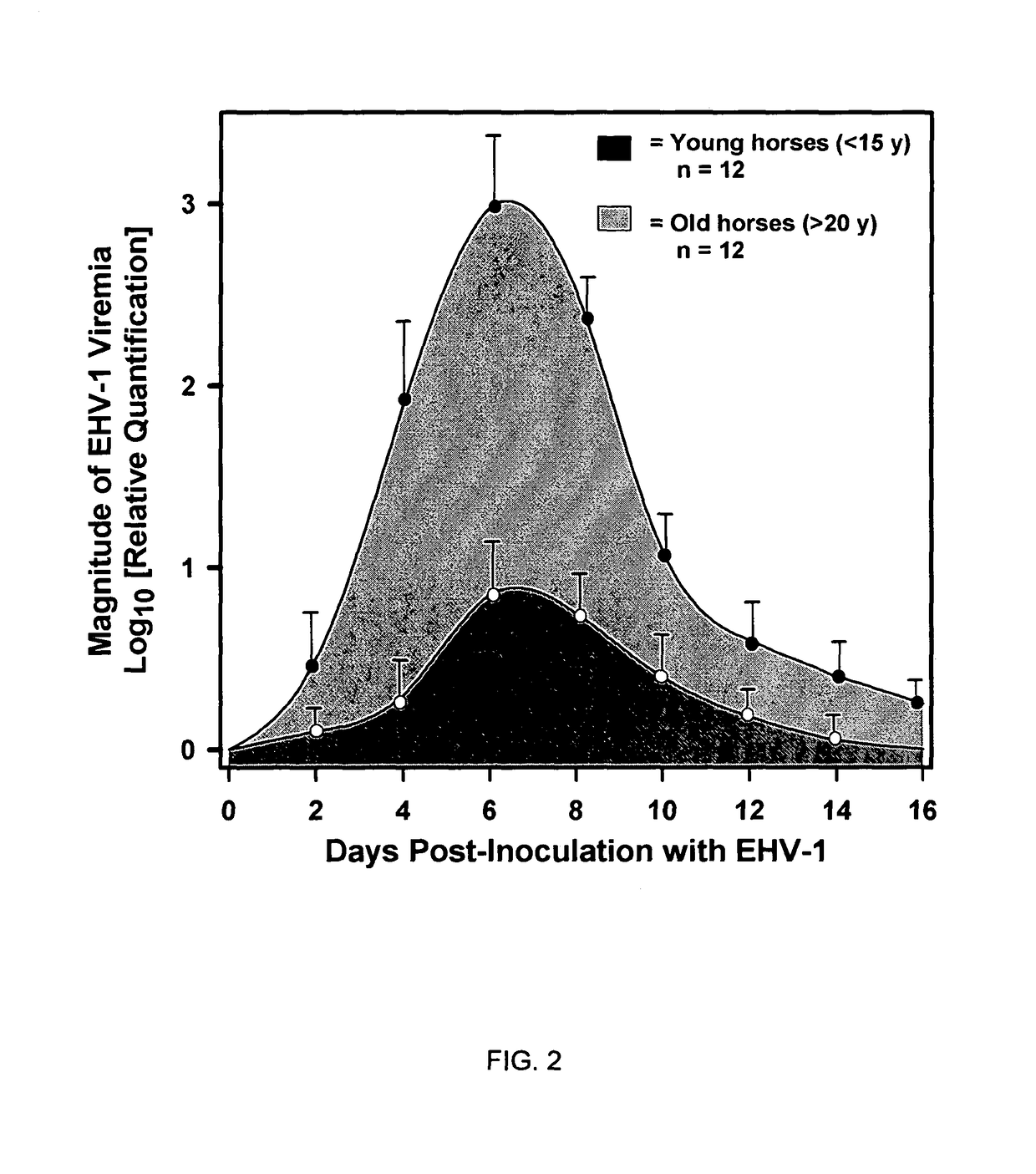
The present invention relates to an in vivo equine disease model for equine herpesvirus-1 neurological disease comprising a horse having a low pre-exposure level of herpesvirus-specific CTL precursors and / or is approximately 20 years of age or older wherein the horse is experimentally infected with a neuropathogenic strain of equine herpesvirus or a mutant thereof. The invention includes a method of preparing an in vivo equine disease model for equine herpesvirus-1 neurological disease comprising obtaining a horse that possesses low pre-infection levels of EHV-1 specific CTL precursors and / or is approximately 20 years of age or older and inoculating the horse intranasally with an effective infecting amount of a neuropathogenic strain of EHV-1. A particularly preferred method involves the advanced neurological disease stage when the experimental horse presents clinical signs of myeloencephalopathy. Additionally, the invention concerns a method of quantifying the risk factors and predicting the development of clinical neurologic signs of equine herpesvirus-1 neurological disease in a horse comprising the steps of (a) determining the pre-infection CTLp frequency to be less than approximately 40 EHV-1 specific CTLp per 106 PBMC; and (b) determining the post-infection viremic load following exposure to EHV-1 to be approximately 10-fold or more over the viremic load present in horses following exposure to a non-neuropathogenic strain of EHV-1. Also described in the invention is the determination of the risk of developing the clinical neurologic signs by use of an equation y=a+bx wherein y is the peak viremic load, a=2.97, b=−0.027 and the variable x is the pre-infection CTLp frequency. Lastly, the invention deals with a new live, attenuated vaccine formulation that is effective against neurologic disease due to equine herpesvirus-1.
Owner:UNIV OF KENTUCKY RES FOUND
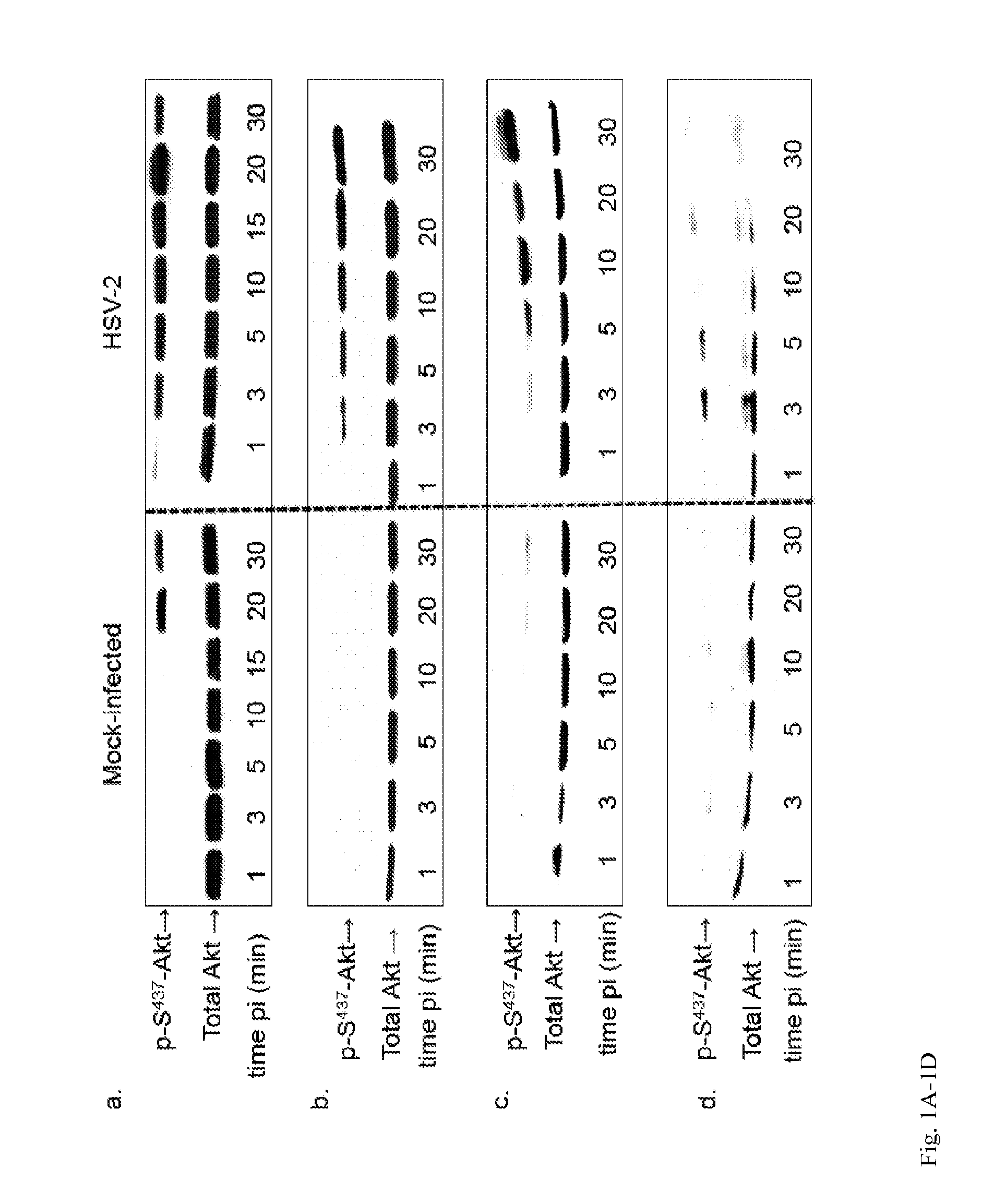
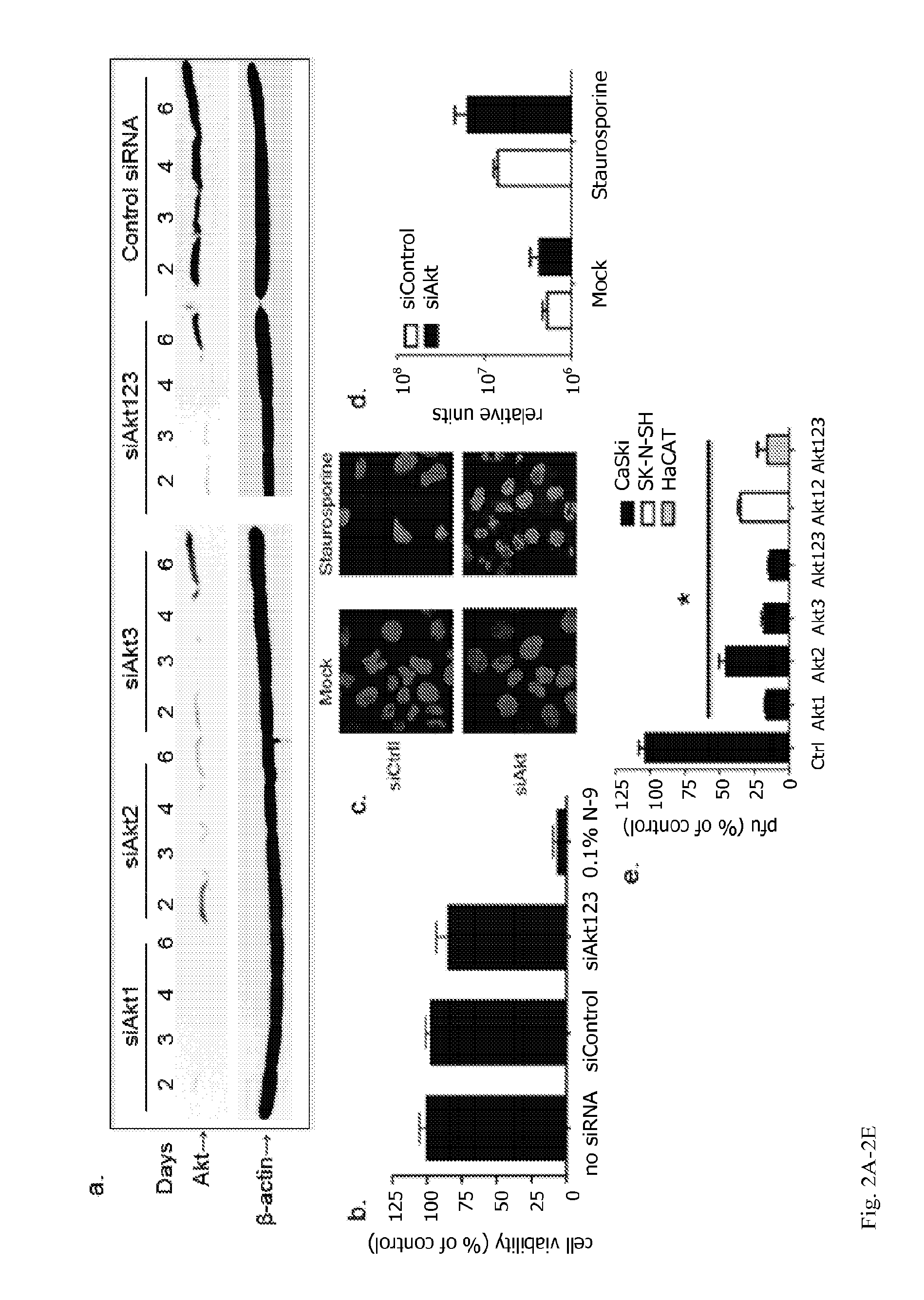
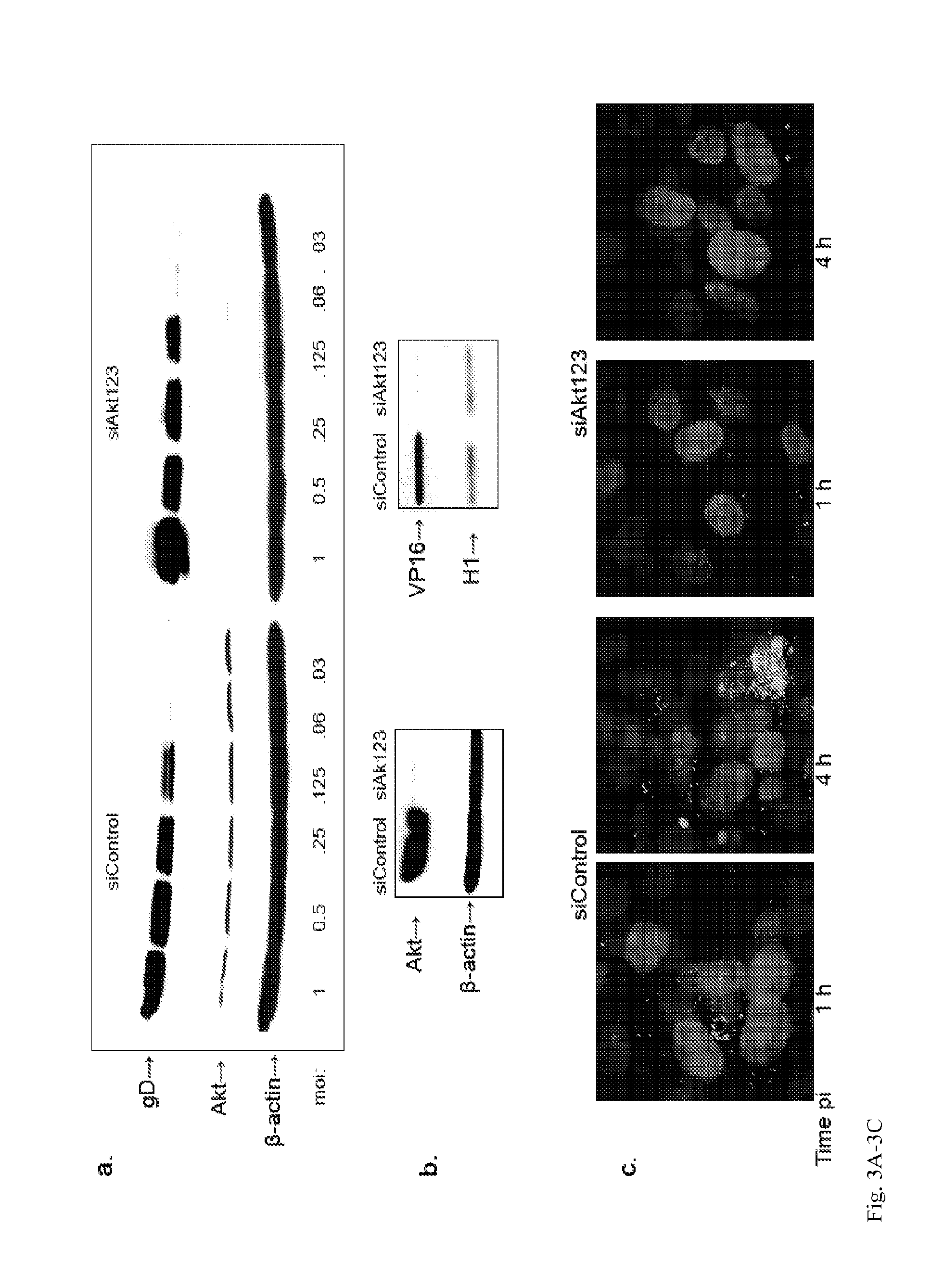
Method to treat or prevent herpesvirus infections
Methods and compositions are provided for inhibiting or treating a herpesvirus infection in a subject using inhibitors of mammalian Akt.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Composition And Method For Inhibiting Herpesviridae Infections
The present invention relates to a pharmaceutical composition when used for inhibiting a herpesviridae infection and / or providing relief from a herpesvindae infection in a subject. The composition includes an effective amount of an extract from a plant in the Asteraceae family.
Owner:VIRATEC
Herpes virus infection inhibitor, method for inhibiting infection with herpes virus, and use thereof
InactiveUS20100129347A1Suppression problemAvoid infectionCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsHerpesvirus infectionHerpes simplex virus DNA
Disclosed are: an inhibitor of herpesvirus infection; a method for the inhibition of herpesvirus infection; and a typical utilization method thereof. The inhibitor of herpesvirus infection comprises an active ingredient which can bind to glycoprotein B or a receptor for glycoprotein B and can inhibit the interaction between glycoprotein B and a receptor for glycoprotein B.
Owner:OSAKA UNIV
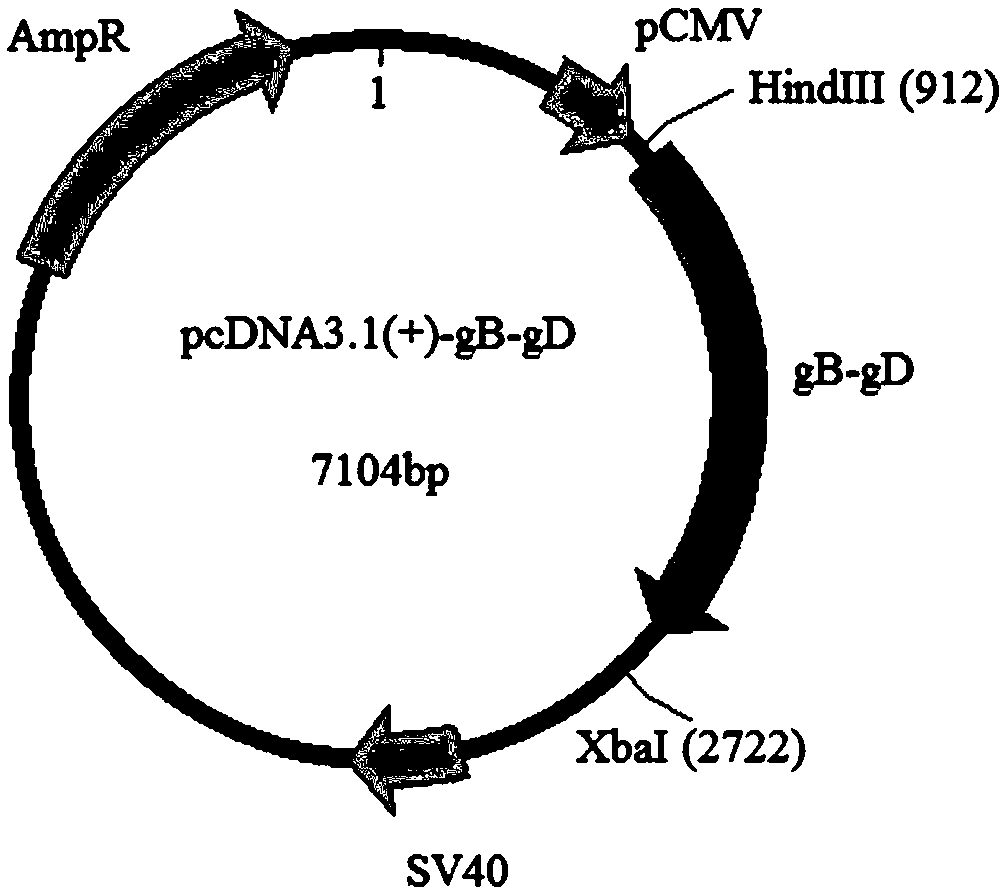
Equine herpesvirus 1 gB-gD fusion protein, recombinant vector, eukaryotic cell strain, preparation method of recombinant vector, and vaccine
ActiveCN109136264AImprove accuracyIncrease success rateAntibody mimetics/scaffoldsViral antigen ingredientsEquine herpesvirus type 1Cell strain
The invention relates to the technical field of biology and particularly provides equine herpesvirus 1 gB-gD fusion protein, a recombinant vector, a eukaryotic cell strain, a preparation method of therecombinant vector, and a vaccine. The equine herpesvirus 1 gB-gD fusion protein recombinant vector provided herein comprises a eukaryotic expression vector fragment and an equine herpesvirus 1 gB-gDgene fragment; the recombinant vector herein allows both equine herpesvirus 1 glycoproteins gB and gD to be expressed in an exogenous system at any time. A construction method provided herein includes: screening CHO (Chinese hamster ovary ) cells containing the recombinant vector are screened to obtain a CHO cell strain that may stably express the equine herpesvirus 1 gB-gD fusion protein; the construction method is simple, easy to perform, and suitable for successful screening of eukaryotic cell strains to stably and highly express eukaryotic equine herpesvirus 1 gB-gD fusion protein; a feasible technical scheme is provided for subsequent various researches, such as vaccine preparation and other applications.
Owner:天康制药股份有限公司
Equine disease model for herpesvirus neurologic disease and uses thereof
InactiveUS20110289606A1Increase chanceProtection levelAntibacterial agentsViral antigen ingredientsNeurological signsEquine herpesvirus
Disclosed is an in vivo equine disease model and a method of preparing the disease model for equine herpesvirus-1 neurological disease comprising a horse having a low pre-exposure level of herpesvirus-specific CTL precursors wherein the horse is experimentally infected with a neuropathogenic strain of equine herpesvirus or a mutant thereof. Also disclosed is a method of quantifying the risk factors and predicting the development of clinical neurologic signs of equine herpesvirus-1 neurological disease in a horse. Also described in the invention is the determination of the risk of developing the clinical neurologic signs by a mathematical equation. A new live, attenuated vaccine formulation is disclosed that is effective against neurologic disease due to equine herpesvirus-1.
Owner:UNIV OF KENTUCKY RES FOUND
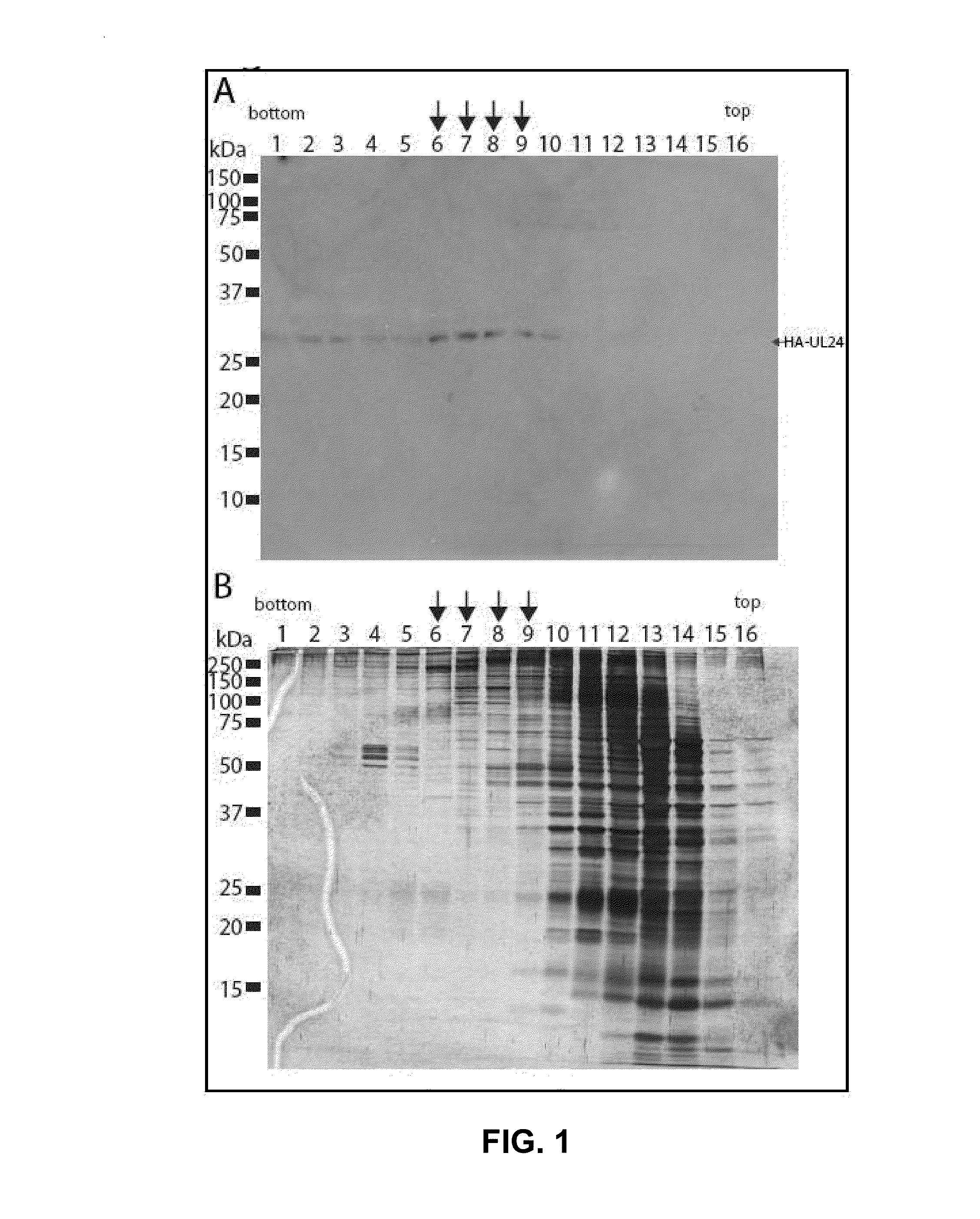
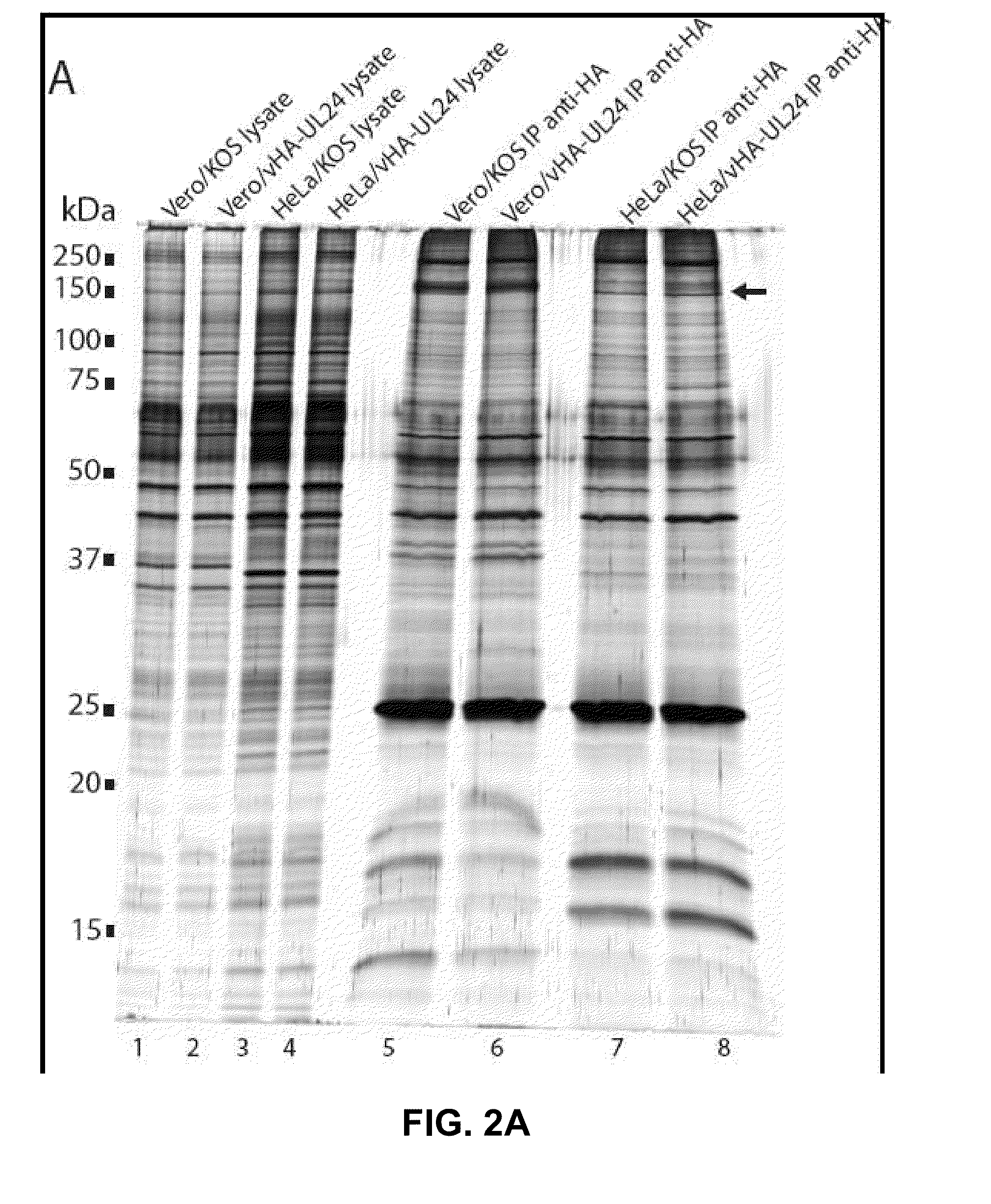
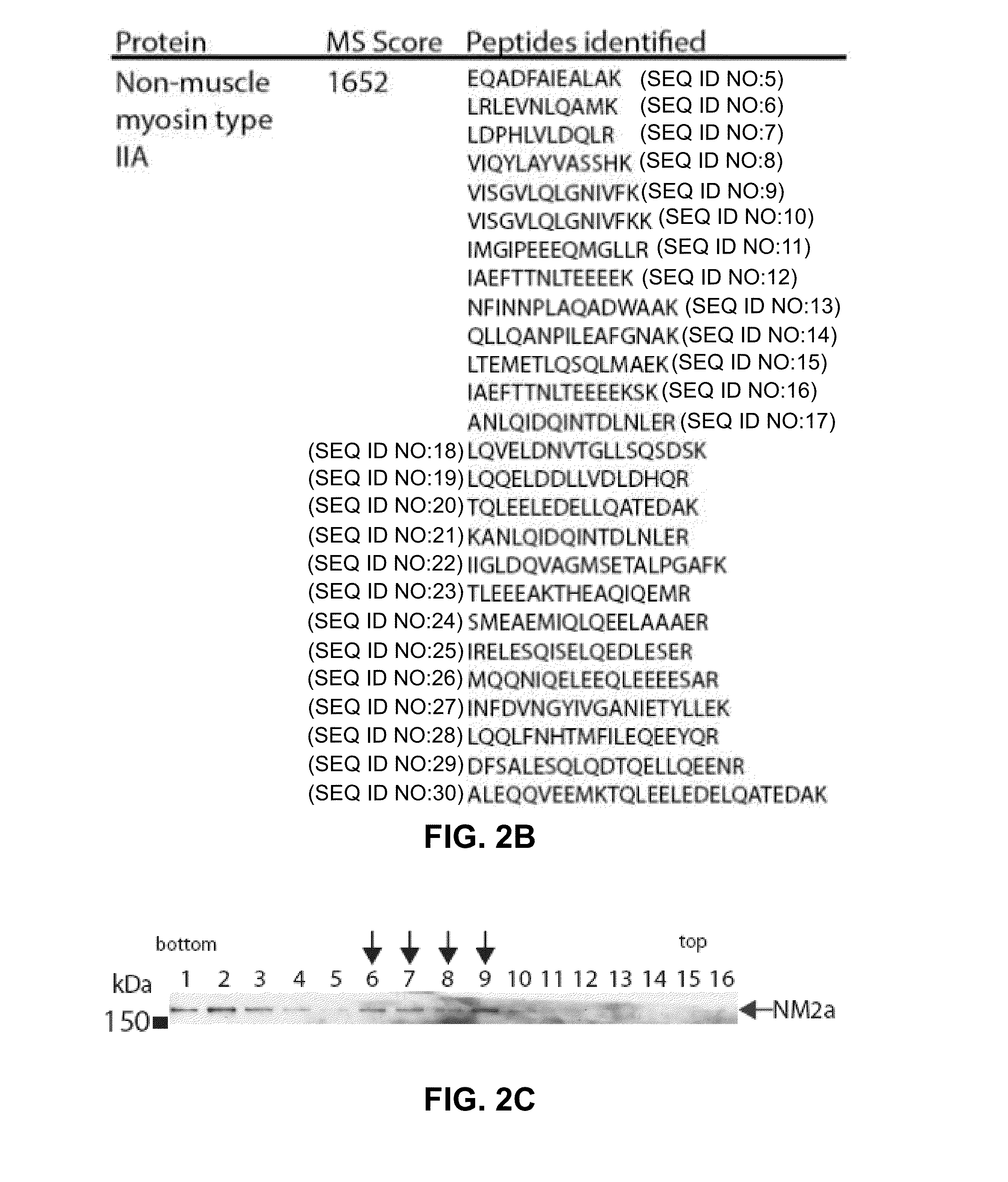
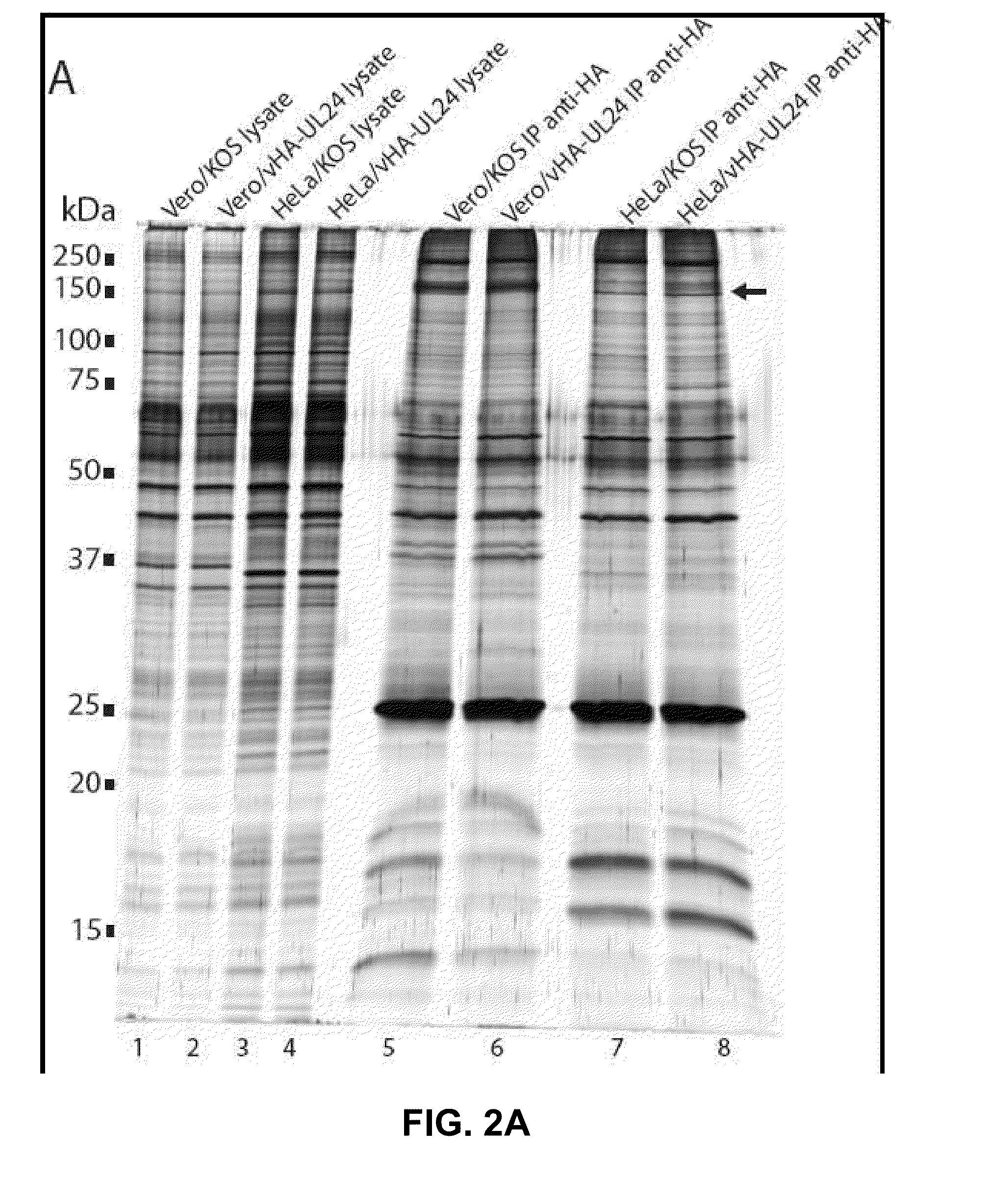
Modulation of ul24 interactions with protein targets and uses thereof for inhibition of herpesvirus infection
Methods and uses for the treatment of herpesvirus infection based on the inhibition of the interaction between herpesvirus UL24 and non-muscle myosin type IIa (NM2a) are described.
Owner:INSTITUT NATIONAL DE LA RECHERCHE SCIENTIFIQUE
Equine herpesvirus-1 and application thereof
PendingCN110885794AGood screening effectEasy to trainMicrobiological testing/measurementDsDNA virusesDiagnostic testA-DNA
The invention relates to equine herpesvirus-1 XJYM2019, protein of the virus and a vaccine based on the virus and protein thereof. The invention also relates to a DNA fragment comprising the gene of the virus and a genetic engineering vaccine based on the gene of the virus. Besides, the invention relates to an antibody reacting with the virus and a diagnostic test for detecting the virus or antibody against the virus.
Owner:XINJIANG AGRI UNIV
Enzyme-linked immunoassay to detect felis catus gammaherpesvirus 1
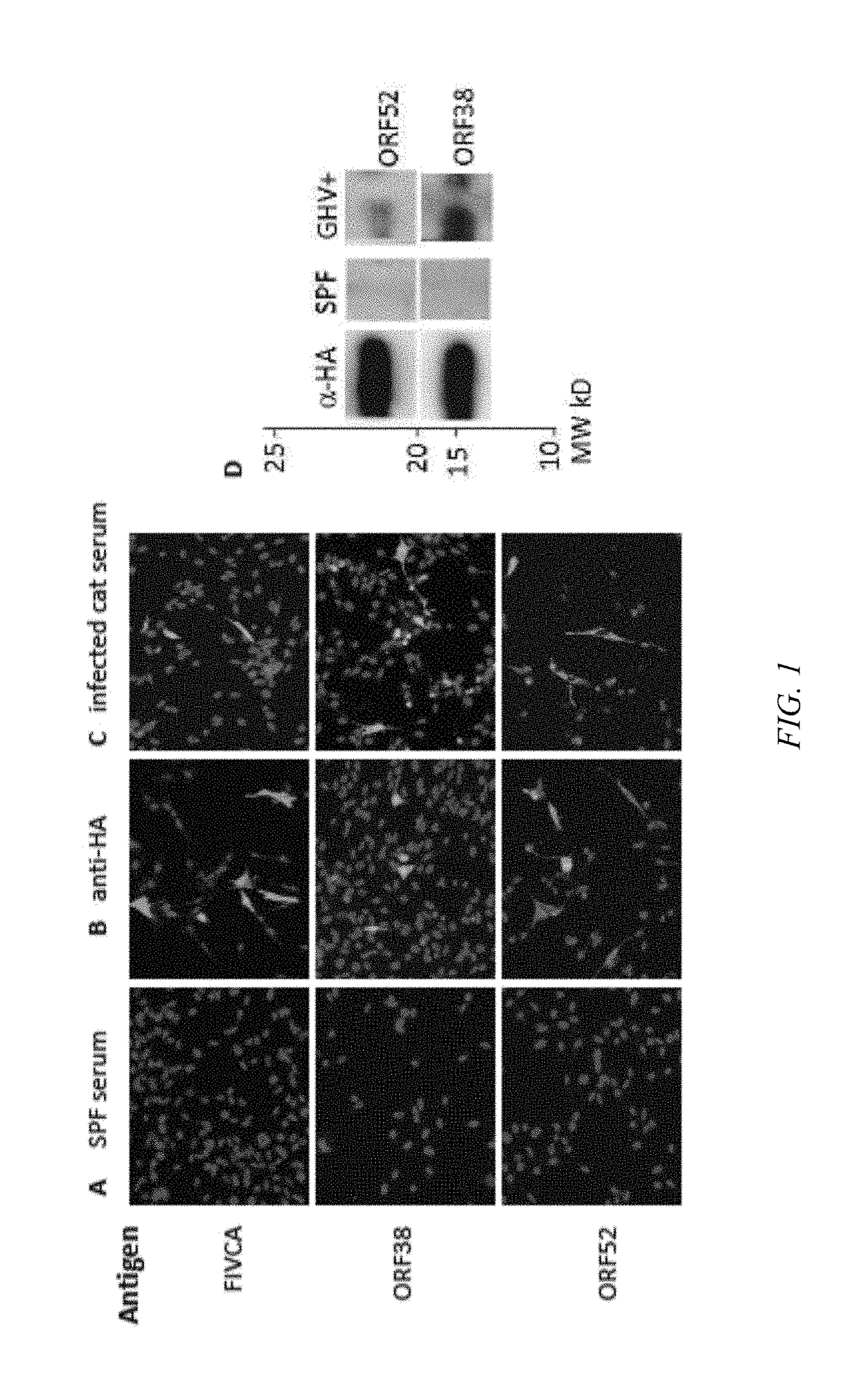
Indirect ELISAs to detect exposure to Felis catus gammaherpesvirus 1 (FcaGHV1) in domestic cats. These ELISAs detect feline serum antibodies to ORF52 and ORF38 of FcaGHV1. The ELISA assays are sensitive, specific, and adaptable for scale up use in high throughput diagnostics.
Owner:COLORADO STATE UNIVERSITY
Microarray-mediated diagnosis of herpes virus infection by monitoring host's differential gene expression upon infection
InactiveCN101065502BMicrobiological testing/measurementBiological testingSequelaHerpes simplex virus DNA
The present invention discloses molecule and test about diseases can be used for diagnosing and evaluating the animals infected by herpes virus, and for testing the animals in herpes virus infection or in sequela. The invention allows early diagnosis and monitoring of an infected animal's immune response and thus enables better treatment and management decisions to be made in clinical and sub-clinically affected animals.
Owner:IMMUNEXPRESS
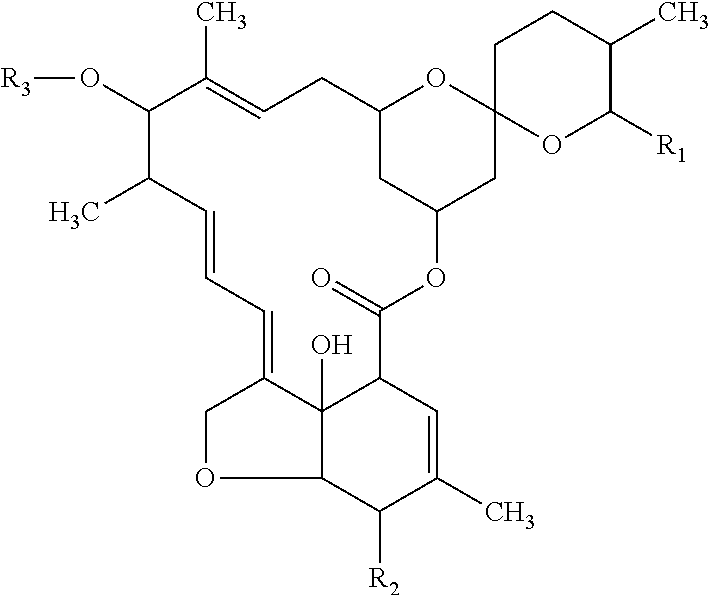
Equine Herpesvirus 1 Vaccine and Vector and Uses Thereof
InactiveUS20130195918A1Decreased lung viral titerReduce synthesisBiocideGenetic material ingredientsEquine herpesvirus 1Mammal
We have constructed a mutant EHV-1 that is lacking the entire 12.7 kbp IR segment of the viral genome and found the mutant EHV-1 to be replication competent, to have the ability to replicate in mammalian cell types (including human cells), and to exhibit reduced virulence in the mouse model of EHV-1 virulence.
Owner:FREE UNIV OF BERLIN +1
Equine herpesvirus type 1 gb-gd fusion protein, recombinant vector and eukaryotic cell strain and preparation method and vaccine thereof
ActiveCN109136264BImprove accuracyIncrease success rateViral antigen ingredientsAntibody mimetics/scaffoldsEquine herpesvirus type 1Herpes simplex virus DNA
The invention relates to the field of biotechnology, and in particular, provides a equine herpesvirus type 1 gB-gD fusion protein, a recombinant vector and a eukaryotic cell strain, a preparation method and a vaccine thereof. The present invention provides a equine herpesvirus type 1 gB-gD fusion protein recombinant vector, comprising a eukaryotic expression vector fragment and a equine herpesvirus type 1 gB-gD gene fragment. The recombinant vector can realize the simultaneous expression of equine herpesvirus type 1 glycoproteins gB and gD in an exogenous system at any time. The construction method provided by the present invention includes screening the CHO cells containing the above-mentioned recombinant vector to obtain a CHO cell strain stably expressing the equine herpes virus type 1 gB-gD fusion protein. The construction method is simple and easy to operate, and can successfully screen out a stable and highly expressing eukaryotic cell line of the equine herpes virus type 1 gB-gD fusion protein, which provides a feasible technical solution for subsequent studies including vaccine preparation and other applications.
Owner:天康制药股份有限公司
Equine disease model for herpesvirus neurologic disease and uses thereof
InactiveUS9642908B2Increase chanceProtection levelAntibacterial agentsPeptide/protein ingredientsNervous systemNeuropathy HIV
Disclosed is an in vivo equine disease model and a method of preparing the disease model for equine herpesvirus-1 neurological disease comprising a horse having a low pre-exposure level of herpesvirus-specific CTL precursors wherein the horse is experimentally infected with a neuropathogenic strain of equine herpesvirus or a mutant thereof. Also disclosed is a method of quantifying the risk factors and predicting the development of clinical neurologic signs of equine herpesvirus-1 neurological disease in a horse. Also described in the invention is the determination of the risk of developing the clinical neurologic signs by a mathematical equation. A new live, attenuated vaccine formulation is disclosed that is effective against neurologic disease due to equine herpesvirus-1.
Owner:UNIV OF KENTUCKY RES FOUND
Modulation of UL24 interactions with protein targets and uses thereof for inhibition of herpesvirus infection
Methods and uses for the treatment of herpesvirus infection based on the inhibition of the interaction between herpesvirus UL24 and non-muscle myosin type IIa (NM2a) are described.
Owner:INSTITUT NATIONAL DE LA RECHERCHE SCIENTIFIQUE
Monoclonal antibody of equine influenza virus nucleoprotein as well as preparation method and application thereof
InactiveCN102242082BHigh ascites titerHigh affinityImmunoglobulins against virusesTissue cultureWestern blotEquine herpesvirus type 1
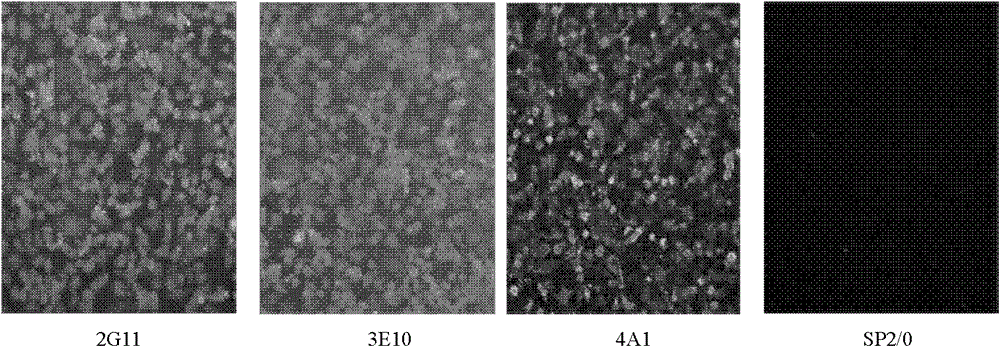
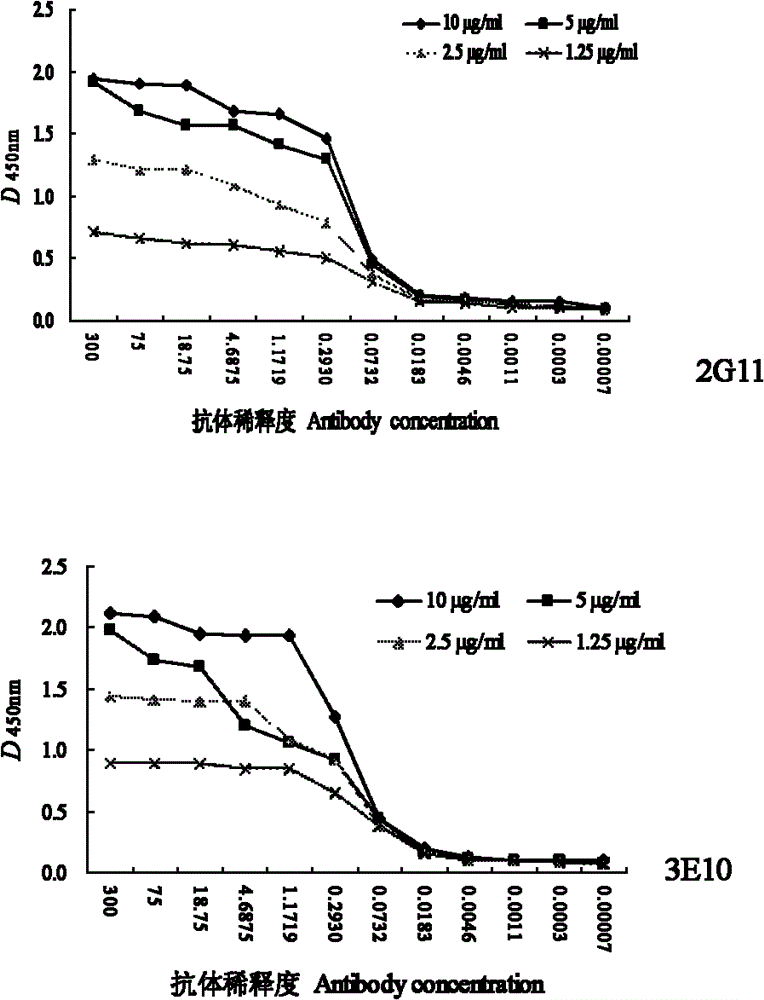
The invention discloses a monoclonal antibody of an equine influenza virus (EIV) nucleoprotein (NP) as well as a preparation method and application thereof. The preparation method comprises the steps of: fusing cells with EIV NP expressed by pronucleus as immunogen, and then cloning the cells by utilizing a limiting dilution method and sifting the cells by utilizing an indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) to obtain two hybridoma strains 2G11 and 3E10 for stably secreting monoclonal antibodies. The two monoclonal antibodies can generate higher ascites titer. Western-blot analysis shows that the two obtained monoclonal antibodies can specifically identify EIV NP recombinant protein; an indirect immunofluorescence experiment proves that the two monoclonal antibodies can be combined with a natural EIV; and cross reaction is not carried out between the two monoclonal antibodies and equine herpesvirus type I, equine herpesvirus type IV and equine encephalitis virus type II through the ELISA. The invention provides the material basis for preparing an anti-EIV NP monoclonal antibody and establishing a subsequent EIV detection method.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method of treating herpes virus infection using macrocyclic lactone compound
A method of treating herpes simplex virus infection or varicella zoster virus infection is disclosed. The method includes topically applying a composition containing an effective amount of one or more macrocyclic lactone compounds, including avermectin compounds or milbemycin compounds and a pharmaceutically acceptable carrier to the affected area of an individual suffering from herpes simplex virus infection or varicella zoster virus infection.
Owner:GALDERMA SA
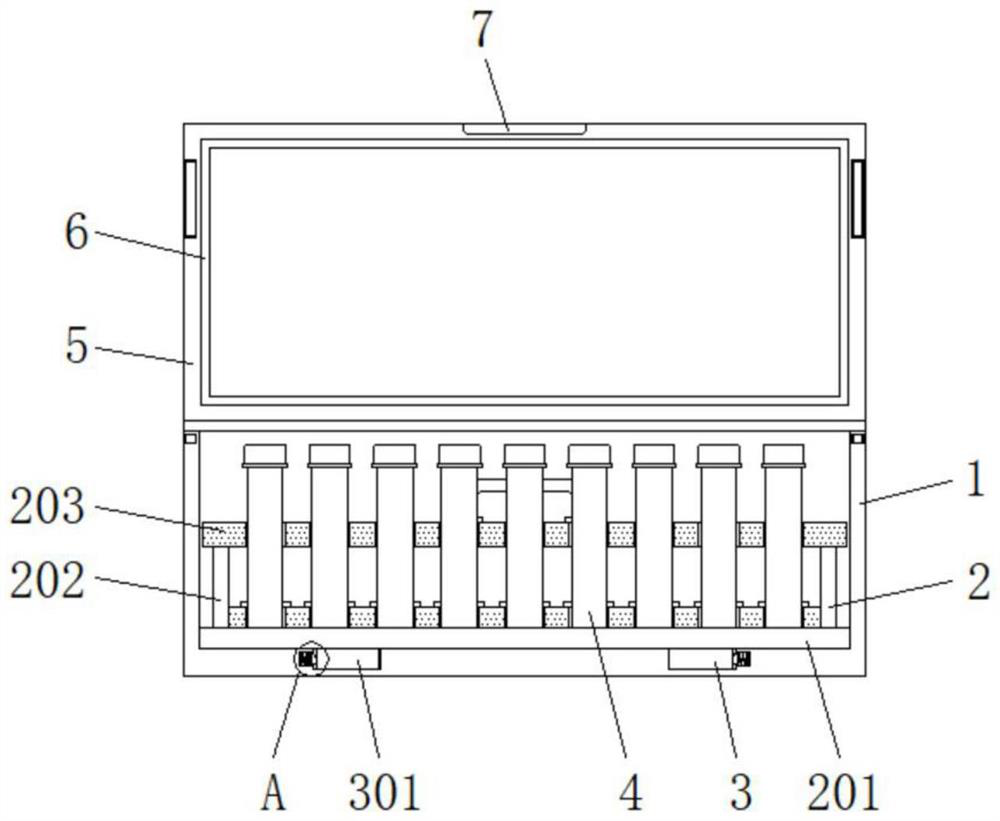
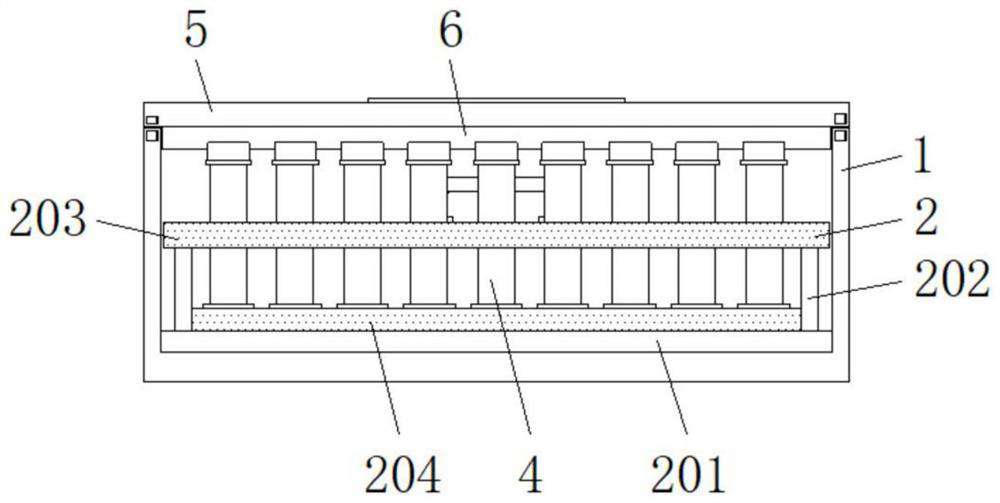
Safe detection kit for detecting I-type and IV-type equine herpes viruses
InactiveCN114572536ANo collisionReduce shakingNon-removalbe lids/coversContainers to prevent mechanical damageStructural engineeringEquine herpesvirus
The invention discloses a safe detection kit for detecting I-type and IV-type horse herpes viruses, the safe detection kit comprises a kit body, a protection structure is arranged in the kit body, the protection structure comprises a bottom plate, the bottom plate is arranged at the bottom end in the kit body, a test tube body is arranged in a limiting hole, and the test tube body is arranged in the limiting hole. A reserved groove is formed in the top of one end of the box cover, and a marking structure is fixed to the other end of the box cover. By arranging the protection structure, when the test tube bodies are placed, the test tube bodies are taken, penetrate through preformed holes and are inserted into limiting holes, the bottoms of the test tube bodies can be limited through the limiting holes, bottle bodies of the test tube bodies can be limited through the preformed holes, and the test tube bodies cannot collide with one another; the test tube bodies can be protected through the limiting holes, shaking of the test tube bodies in the limiting holes is reduced, all the test tube bodies can be conveniently taken out of the kit body by lifting the handle upwards, and the convenience of the kit is improved.
Owner:徐州元盛生物科技有限公司
Methods of Treating Herpesvirus Infections
InactiveUS20140227281A1Avoid infectionAvoid spreadingBiocidePeptide/protein ingredientsHeavy chainHerpesvirus infection
Owner:THE UNIV OF TOKYO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com