Application of polycyclic polyketides in preparation of anti-herpesvirus drugs
An anti-herpes virus and polyketide compound technology, applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as easy mutation, high drug resistance, and low protein quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
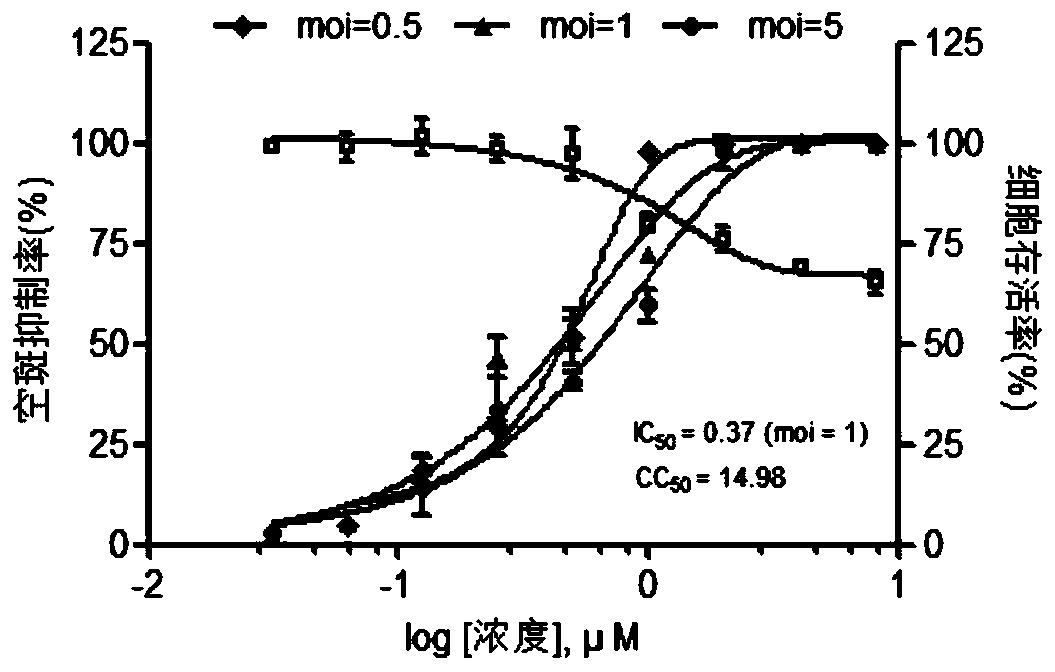
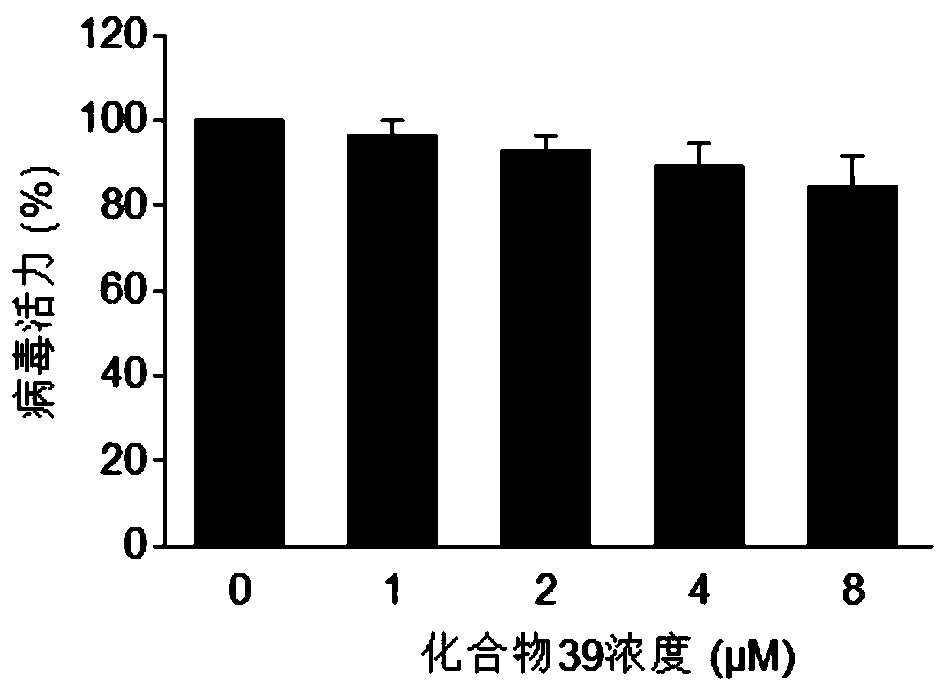
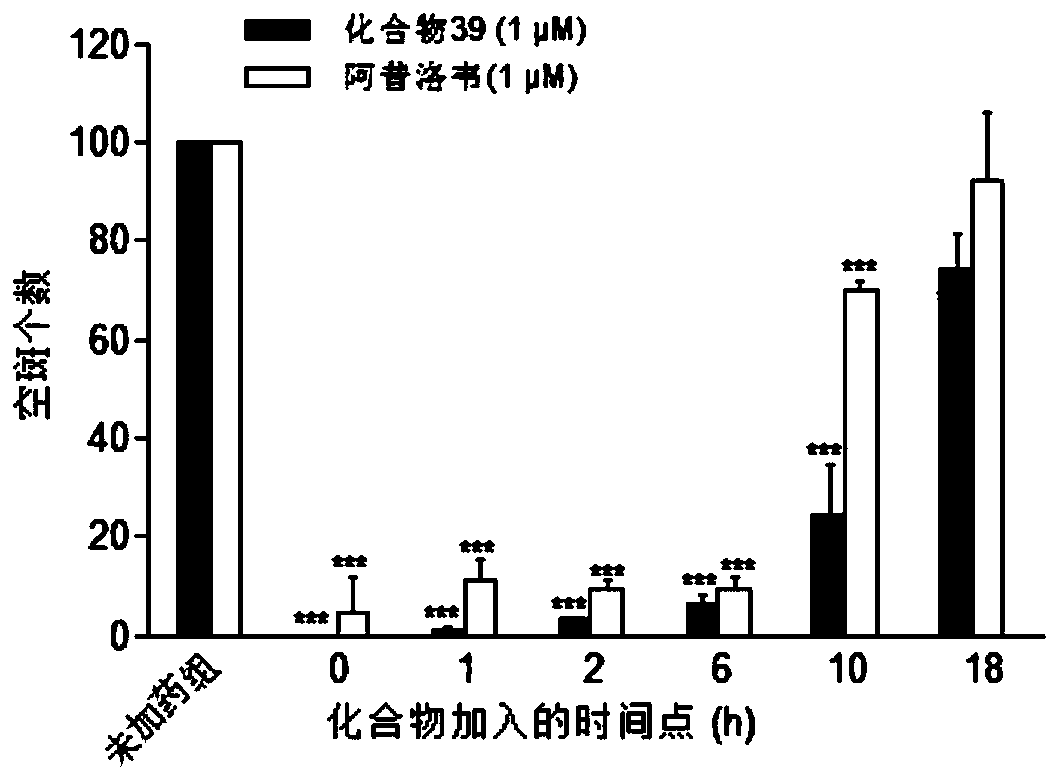
Image
Examples
Embodiment 1
[0092] 6,8-dihydroxy-9-isobutyl-2,2,4,4-tetramethyl-4,9-dihydro-1H-xanthene-1,3(2H)-dione intermediate ( 52) Preparation
[0093]
[0094] first step
[0095] At room temperature, dissolve phloroglucinol 52a (2.5g, 20mmol) in nitromethane solution, add anhydrous aluminum chloride (10.7g, 80mmol) and isobutyryl chloride (2.3g, 22mmol) successively, and heat up to reflow. After reacting for 12 hours, the reaction solution was slowly poured into ice water, and saturated sodium potassium tartrate solution (100 mL) was added, followed by vigorous stirring. The reaction solution was extracted 3 times with ethyl acetate (100 mL×3). The organic phases were combined and washed with saturated NaCl solution. After drying and filtering over anhydrous sodium sulfate, the organic phase was evaporated to dryness under reduced pressure. The resulting crude product was separated and purified by silica gel column chromatography to obtain compound 52c (3.6 g, yield 85%).
[0096] second...
Embodiment 2
[0104] 6,8-Dihydroxy-9-cyclobutyl-2,2,4,4-tetramethyl-4,9-dihydro-1H-xanthene-1,3(2H)-dione intermediate ( 53) Preparation
[0105]
[0106] first step
[0107] At room temperature, dissolve phloroglucinol 52a (2.5g, 20mmol) in nitromethane solution, add anhydrous aluminum chloride (10.7g, 80mmol) and cyclobutyryl chloride (2.6g, 22mmol) successively, and heat up to reflow. After reacting for 12 hours, the reaction solution was slowly poured into ice water, and saturated sodium potassium tartrate solution (100 mL) was added, followed by vigorous stirring. The reaction solution was extracted 3 times with ethyl acetate (100 mL×3). The organic phases were combined and washed with saturated NaCl solution. After drying and filtering over anhydrous sodium sulfate, the organic phase was evaporated to dryness under reduced pressure. The obtained crude product was separated and purified by silica gel column chromatography to obtain compound 53c (3.4 g, yield 83%).
[0108] sec...
Embodiment 3
[0116] 6,8-Dihydroxy-5-acetyl-9-isobutyl-2,2,4,4-tetramethyl-4,9-dihydro-1H-oxanthene-1,3(2H)- Preparation of diketone (5)
[0117] At room temperature, compound 52 (44.2 mg, 0.1 mmol) was dissolved in glacial acetic acid solution (4 mL), acetic anhydride (20.6 μL, 0.22 mmol) and boron trifluoride diethyl ether (13.6 μL, 0.105 mmol) were added, and the temperature was raised to 100°C. After reacting for 3 hours, the reaction was cooled down to room temperature, 1N aqueous sodium hydroxide solution (4 mL) was added to quench the reaction, and extracted three times with ethyl acetate (8 mL×3). The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain a crude product. The obtained crude product was recrystallized in a mixture of dichloromethane and n-hexane to obtain compound 5 (16.0 mg, yield 40%).
[0118] 1 H NMR (500MHz, CDCl 3 )δ13.54(s,1H),7.09(s,1H),6.28(s,1H),4.33(t,J=6.1Hz,1H),2.82(s,3H),1.65(s,3H), 1.50(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com