Equine herpesvirus 1 gB-gD fusion protein, recombinant vector, eukaryotic cell strain, preparation method of recombinant vector, and vaccine
A equine herpes virus and fusion protein technology, applied in the directions of virus/bacteriophage, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as no equine rhinopneumonia can be prevented, and achieve good recombination effect, good stability, Effects with high accuracy and success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The present invention provides a method for preparing the above equine herpesvirus type 1 gB-gD fusion protein recombinant vector, comprising the following steps: inserting the equine herpesvirus type 1 gB-gD gene fragment into the eukaryotic expression vector fragment, and recombining to obtain Equine herpesvirus type 1 gB-gD fusion protein recombinant vector.
[0052] The method is simple and easy to operate, has good recombination effect, and high accuracy and success rate.
[0053] In a preferred embodiment of the present invention, the preparation method includes the following steps: separately processing the equine herpesvirus type 1 gB-gD gene fragment and the eukaryotic expression vector fragment to obtain cohesive ends, and then performing enzyme ligation to obtain equine herpesvirus type 1 gB -gD fusion protein recombinant vector. The double restriction sites at both ends of the eukaryotic expression vector fragment and the equine herpesvirus type 1 gB-gD gen...
Embodiment 1
[0086] Example 1 Construction of pcDNA3.1-gB-gD recombinant plasmid
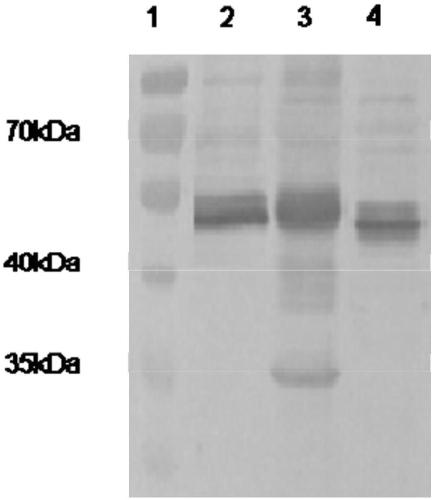
[0087] The schematic diagram of pcDNA3.1-gB-gD recombinant plasmid spectrum is as follows figure 1 shown.
[0088]1.1 After optimization, the gB gene (GeneID: 1487545) is designed to be 720bp in length, and the gD gene (GeneID: 2948561) is optimized to be 747bp in length. The gB and gD gene fragments are connected with the sequence 5'-ggaggaggaggatctggaggaggaggatct-3' to obtain the target fragment gB-gD gene, the full length of the target fragment is 1497bp. Restriction sites were designed upstream and downstream: HindⅢ, XbaI. The target fragment sequence is shown in Figure SEQ ID NO.1.
[0089] The synthesis of target fragments with enzyme cleavage sites was entrusted to Shanghai Sangon Biotechnology Co., Ltd.
[0090] 1.2 gB-gD gene and vector were subjected to double enzyme digestion reaction
[0091] 1.2.1 Construct a 50 μL reaction system: 5 μL of 10× buffer, 2 μg of DNA sample, 2.5 μL of HindⅢ, 2....
Embodiment 2
[0145] Example 2 Transfection of CHO cells and screening of monoclonal eukaryotic cell lines expressing equine herpesvirus type 1 gB-gD fusion protein
[0146] 2.1 Transfection of CHO-K1 cells
[0147] 1) Take out the cells, discard the supernatant medium, wash once with pre-warmed 8mL PBS, and discard the PBS;
[0148] 2) Add 2 mL of 0.25% trypsin-EDTA to each culture dish, digest at room temperature for 2 minutes, and observe under the microscope that the cells become round and appear as single cells;
[0149] 3) Add 4mL DMEM / F12 (containing 10% serum, 1% double antibody) to terminate the digestion reaction, and blow the cells away with a pipette;
[0150] 4) Transfer to a 15mL centrifuge tube and centrifuge at 200rpm for 5min;
[0151]5) DMEM / F12 (containing 10% serum, 1% double antibody) resuspended cells and counted;
[0152] 6) Dilute cells to 2×10 5 cells / mL, take 2mL of mixed cells and add them to a six-well culture dish, place at 37°C, 5% CO 2 Incubate overnight ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com