Cmv glycoproteins and recombinant vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evasion of CD8+ T Cells is Critical for Superinfection by Cytomegalovirus
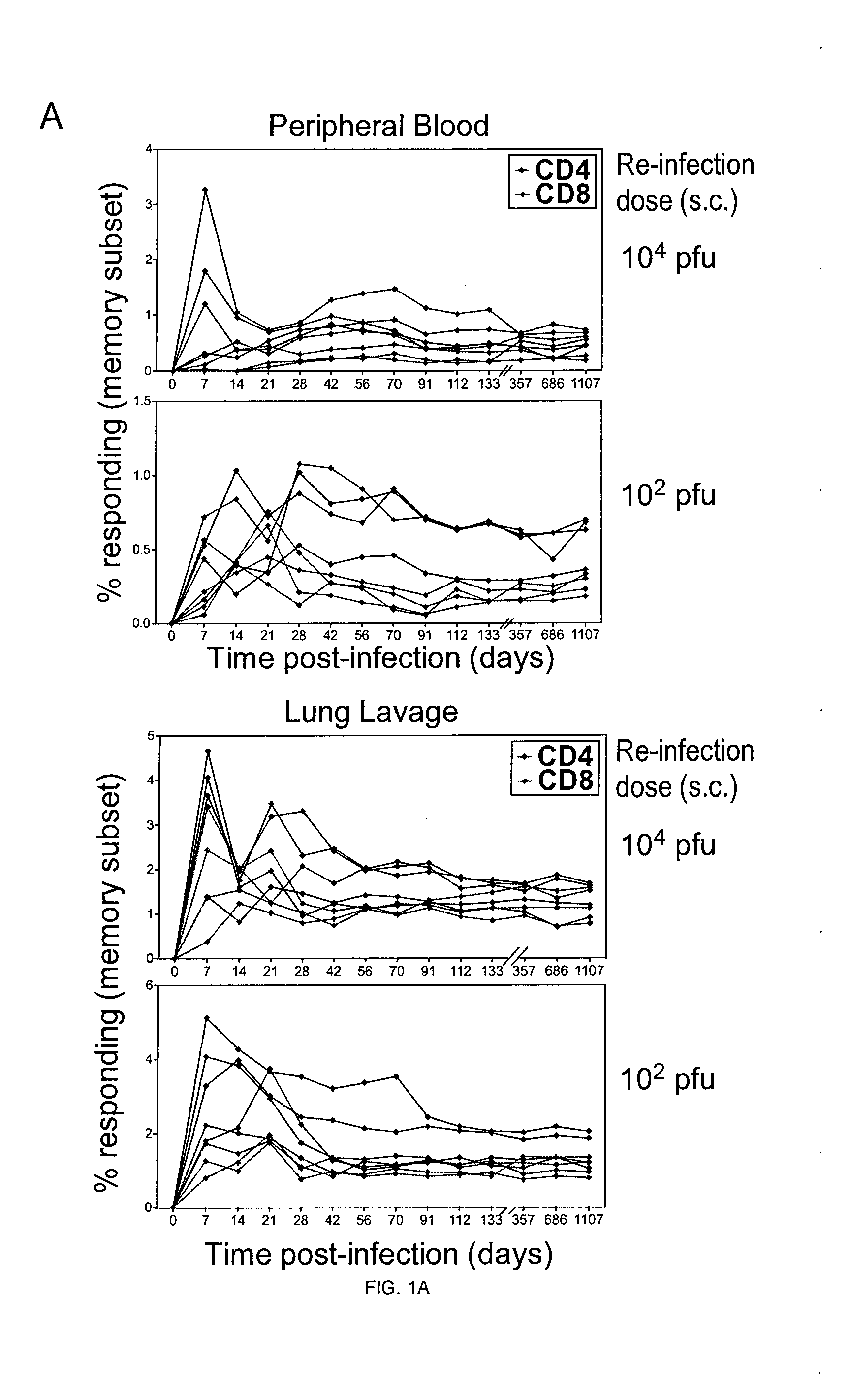
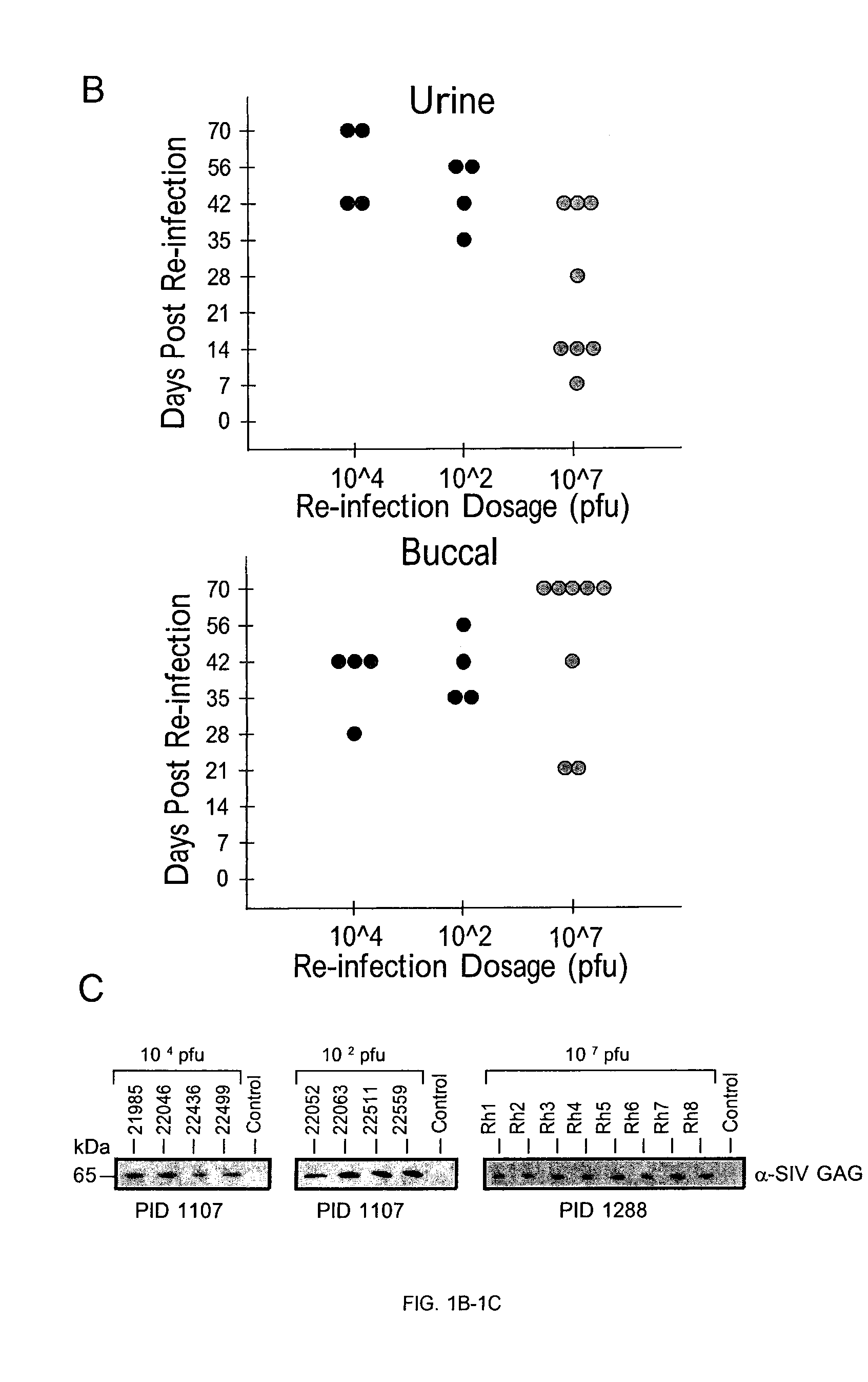
[0214]Cytomegalovirus (CMV) may superinfect persistently infected hosts despite CMV-specific humoral and cellular immunity; however, how it does so remains undefined. Applicants have demonstrated that superinfection of rhesus CMV-infected rhesus macaques (RM) requires evasion of CD8+ T cell immunity by virally encoded inhibitors of major histocompatibility complex class I (MHC-I) antigen presentation, particularly the homologs of human CMV US2, 3, 6, and 11. In contrast, MHC-I interference was dispensable for primary infection of RM, or for the establishment of a persistent secondary infection in CMV-infected RM transiently depleted of CD8+ lymphocytes. These findings demonstrate that US2-11 glycoproteins promote evasion of CD8+ T cells in vivo, thus supporting viral replication and dissemination during superinfection, a process that complicates the development of preventive CMV vaccines but that may be exploit...
example 2
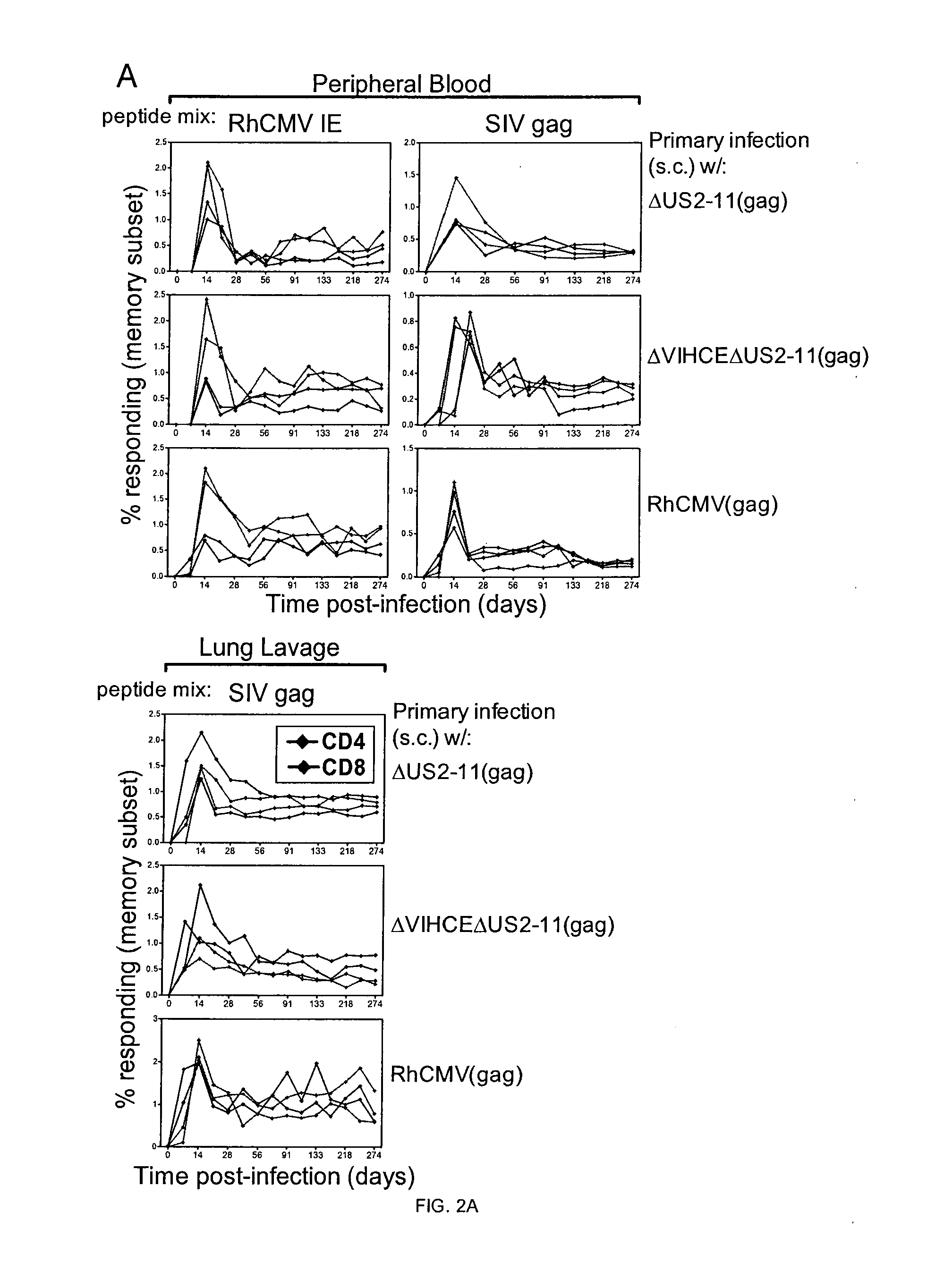
[0240]In this Example, Applicants develop a number of attenuated RhCMV-vaccines to examine the highest level of attenuation that may still achieve protection against ΔUS2-11-Gag. A limitation of Applicants' preliminary data was that Applicants had only shown that natural infection with RhCMV was protective against re-infection with ΔUS2-11, but Applicants had yet to demonstrate that experimental infection with recombinant RhCMV would be protective. Applicants now demonstrate that a recombinant virus lacking the major tegument proteins pp65a and pp65b or pp71 protects against re-infection by ΔUS2-11-Gag.
[0241]PP65 is one of the most abundant proteins in HCMV particles and the most abundant component of the viral tegument, an amorphous protein structure layered between the capsid and the envelope. In addition to its role in evading innate immune responses, pp65 is one of the most immunogenic proteins encoded by HCMV and it is therefore included in most experimental vaccines and pp65-s...
example 3
A Systematic Evaluation of Cytomegalovirus Vaccine Efficacy
[0257]Although human cytomegalovirus (HCMV) causes a mostly benign, unnoticed persistent infection in immunocompetent individuals, it may cause disease in immunocompromised individuals such as transplant or AIDS patients. HCMV is also the most frequent infectious cause of birth defects, with an estimated 0.7% of babies in the APPLICANTS being born with congenital infection, and approximately 10% of these infections resulting in long-term sequelae (primarily sensorineural defects) (Dollard, S. C. et al. 2007. Rev Med Virol 17:355-63). The annual health costs to care for these children is estimated to be about $1-2 billion (Cannon, M. J., and K. F. Davis. 2005. BMC Public Health 5:70). For these reasons, the development of a CMV vaccine has been given high priority by the Institute of Medicine and the National Vaccine Advisory Committee (Arvin, A. M. et al. 2004. Clin Infect Dis 39:233-9). However, the development of a vaccine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com