Intravenous injection of cytomegalovirus human immunoglobulin and its preparation method
A technology for human immunoglobulin and cytomegalovirus, which is applied in the field of human immunoglobulin and its preparation, can solve the problems of low efficiency of ethanol precipitation and purification, high hardware and operating costs, low yield in the process, and reduce energy consumption. and labor intensity, improve product purity, improve the effect of purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
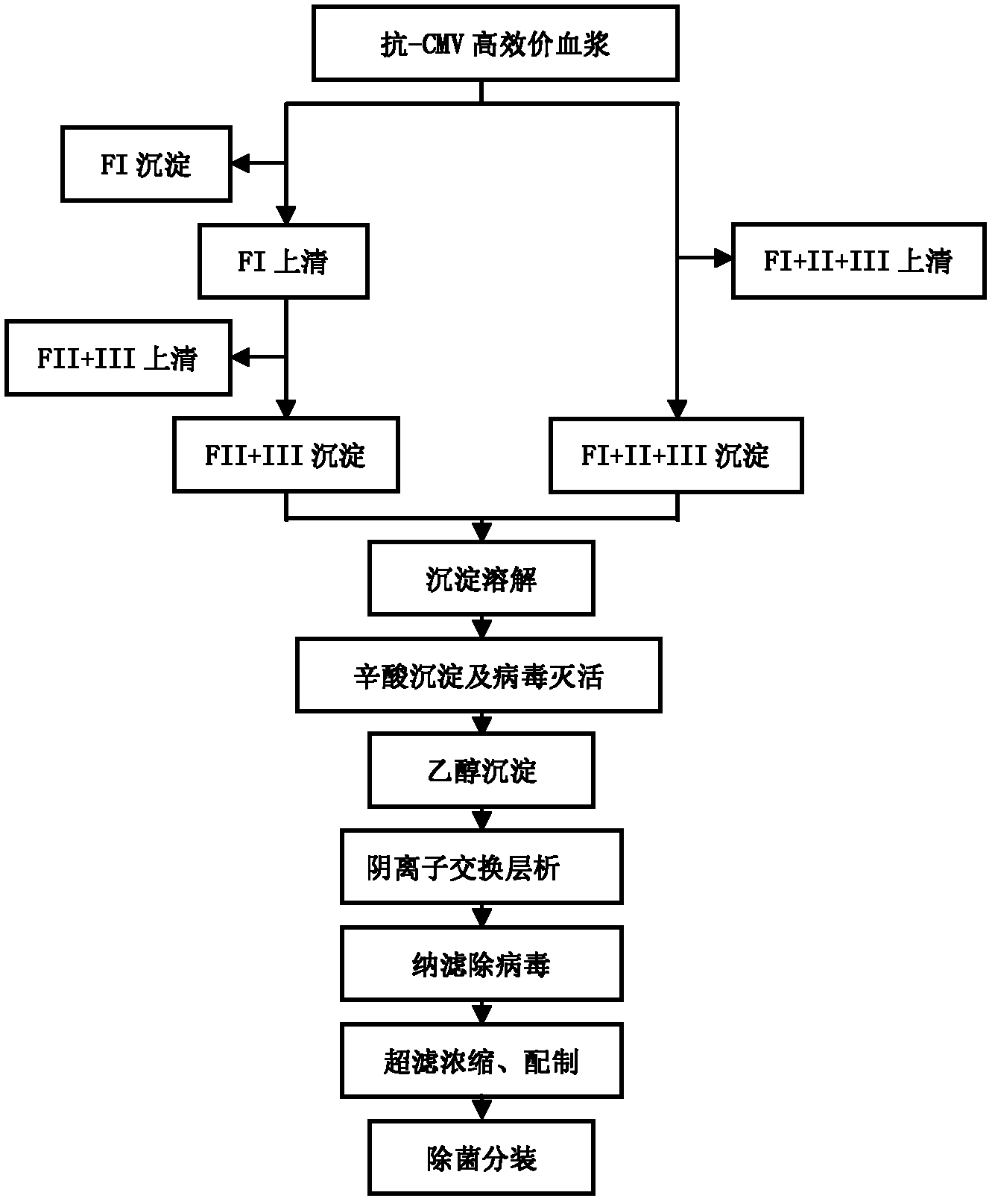
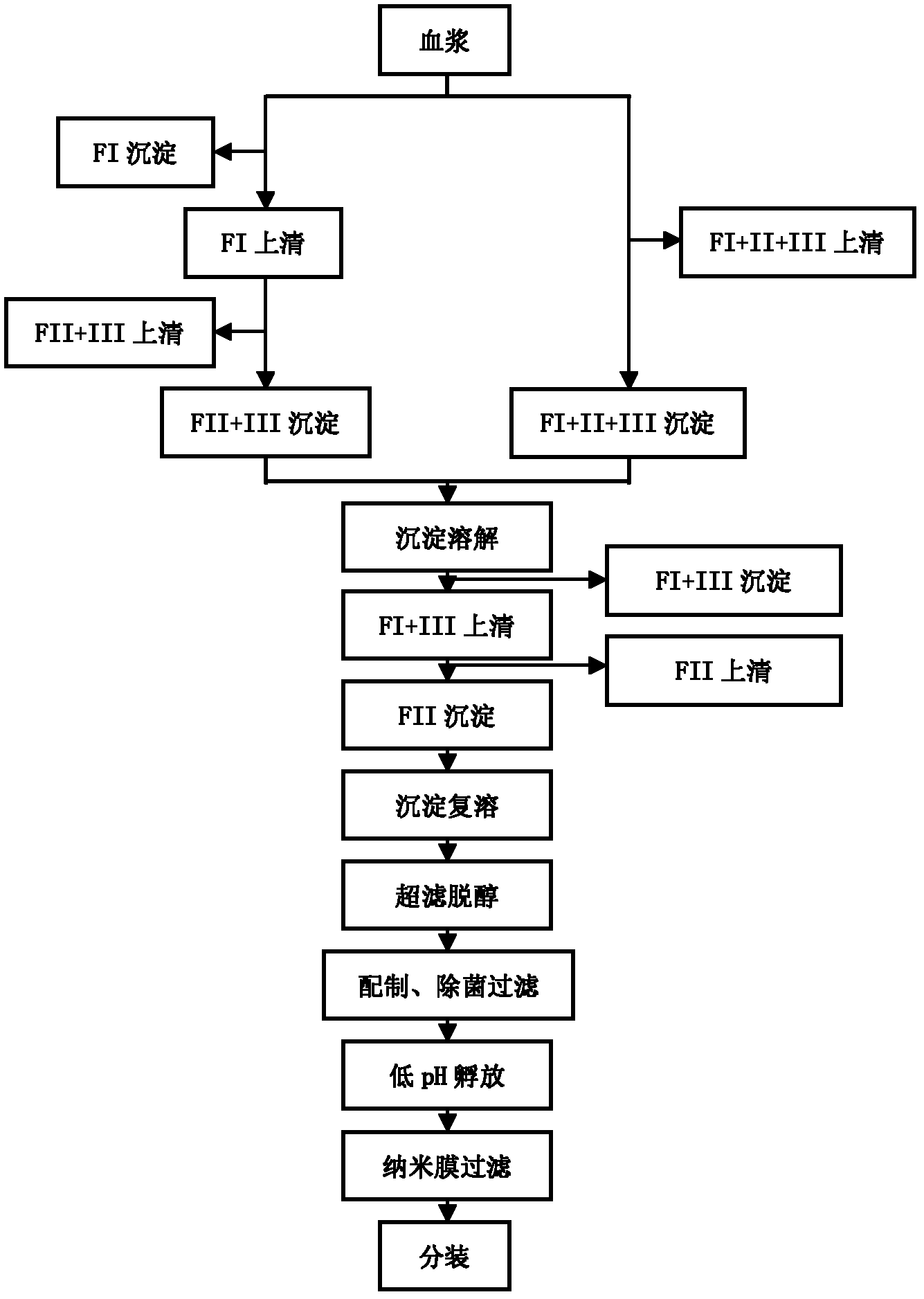
[0050] Example 1, such as figure 1 as shown,
[0051] (1) Take 20 portions of human plasma whose anti-CMV high titer was determined by enzyme-linked immunosorbent assay, thaw the slurry at 25°C, and the volume after mixing is 11540ml.
[0052] (2) Add 2310ml of normal saline to adjust the plasma protein content to 49.53mg / ml, add glacial acetic acid to adjust the pH to 6.18, add 3926ml of absolute ethanol to adjust the ethanol concentration of the suspension to 22%, adjust the reaction temperature to -5.0°C, and stir the reaction After 4 hours, the reaction was completed and centrifuged to obtain FI+II+III precipitates.
[0053] (3) FI+II+III precipitate was dissolved in 11500 ml of sodium acetate buffer solution with a pH of 4.93 and a concentration of 50 mmol / L, stirred at 4° C. for 12 hours, and centrifuged to separate the supernatant.
[0054] (4) Adjust the pH of the supernatant to 4.57 with 4 mol / L acetic acid, add octanoic acid at a concentration of 60 mmol / L, stir an...
Embodiment 2
[0066] (1) Take 20 portions of human plasma whose anti-CMV high titer was determined by enzyme-linked immunosorbent assay, thaw the slurry at 30°C, and the volume after mixing is 11410ml.
[0067] (2) Add 2280ml of physiological saline to adjust the plasma protein content to 50.30mg / ml, add glacial acetic acid to adjust the pH to 6.48, add 4603ml of absolute ethanol to adjust the ethanol concentration of the suspension to 25%, adjust the reaction temperature to -4.5°C, and stir for 6 Hours, the reaction was completed and centrifuged to obtain FI+II+III precipitates.
[0068] (3) FI+II+III precipitate was dissolved in 10500ml of sodium acetate buffer solution with a pH of 5.16 and a concentration of 50mmol / L, stirred at 2.2°C for 12 hours, and the supernatant was separated by centrifugation.
[0069] (4) Adjust the pH of the supernatant to 5.07 with 4 mol / L acetic acid, add octanoic acid at a concentration of 30 mmol / L, stir at 25° C. for 2.5 hours, and centrifuge the supernata...
Embodiment 3
[0082] (1) Take 20 portions of human plasma whose anti-CMV high titer was determined by enzyme-linked immunosorbent assay, and thaw the plasma at 15°C, and the volume after mixing is 11570ml.
[0083] (2) Add 2310ml of normal saline to adjust the plasma protein content to 49.69mg / ml, add glacial acetic acid to adjust the pH to 6.32, add 4603ml of absolute ethanol to adjust the ethanol concentration of the suspension to 20%, adjust the reaction temperature to -4.8°C, and stir for 6 hours After the reaction is completed, centrifuge to obtain FI+II+III precipitate.
[0084] (3) The FI+II+III precipitate was dissolved in 12500 ml of sodium acetate buffer solution with a pH of 5.02 and a concentration of 80 mmol / L, stirred at 6.0° C. for 14 hours, and the supernatant was separated by centrifugation.
[0085] (4) Adjust the pH of the supernatant to 5.50 with 1 mol / L sodium hydroxide, add octanoic acid at a concentration of 40 mmol / L, stir at 23° C. for 1 hour, and centrifuge the sup...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com