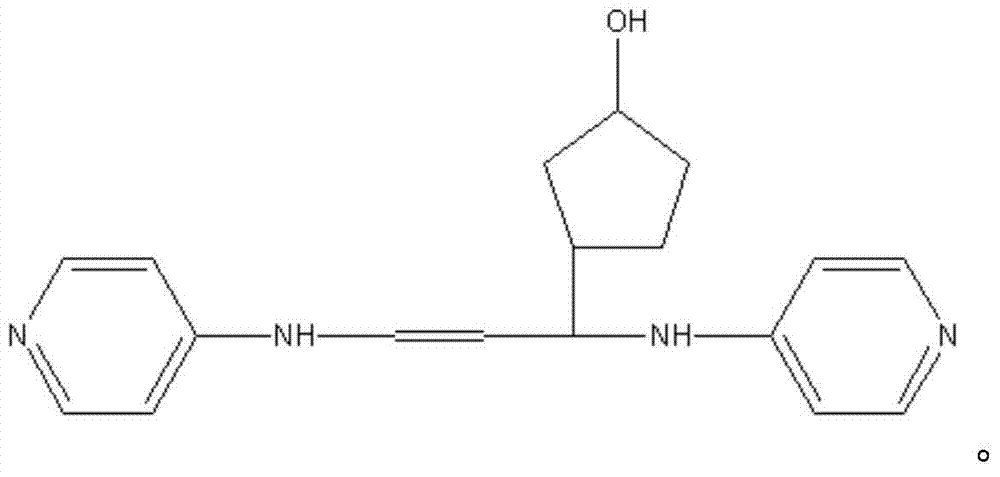
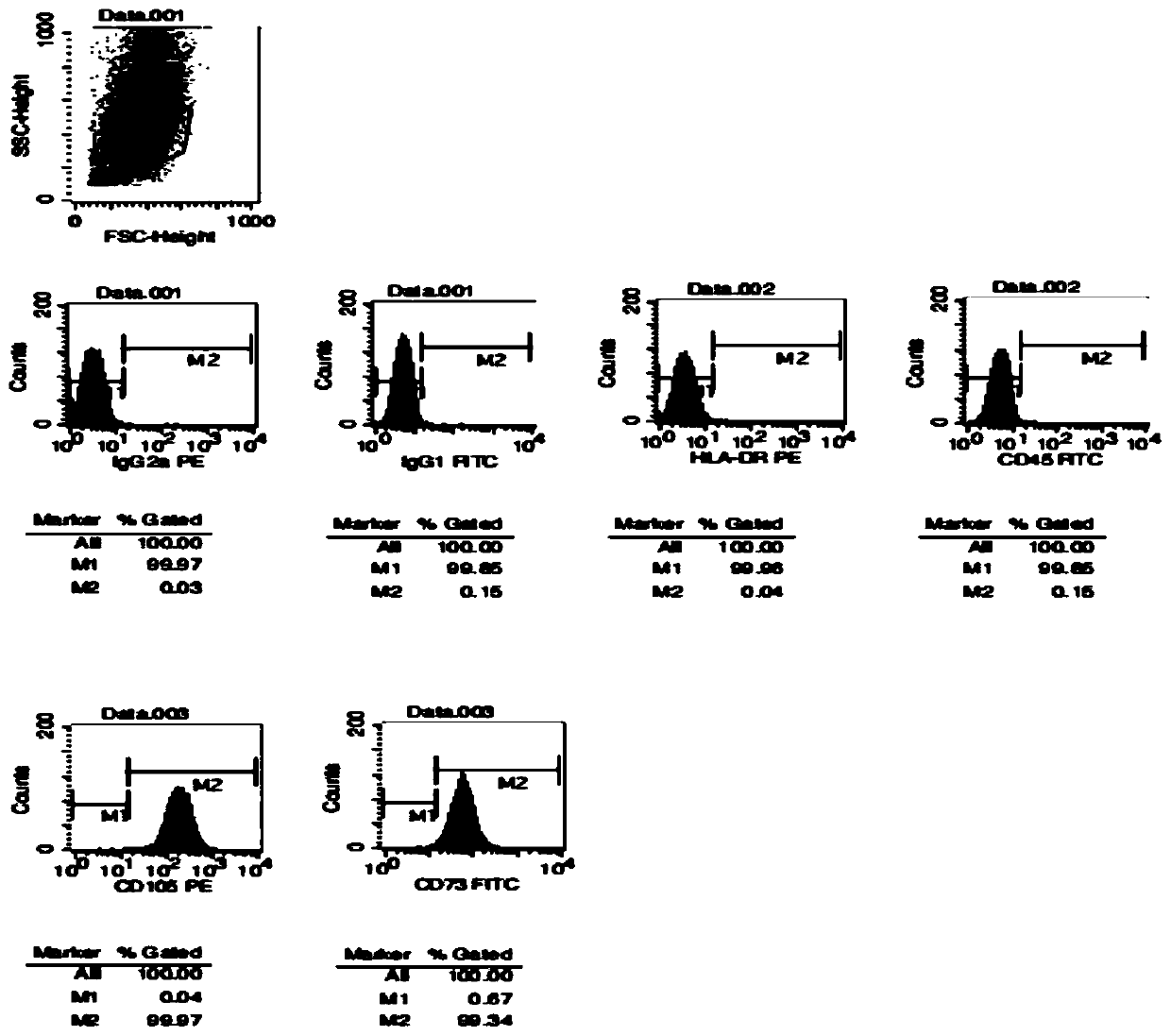
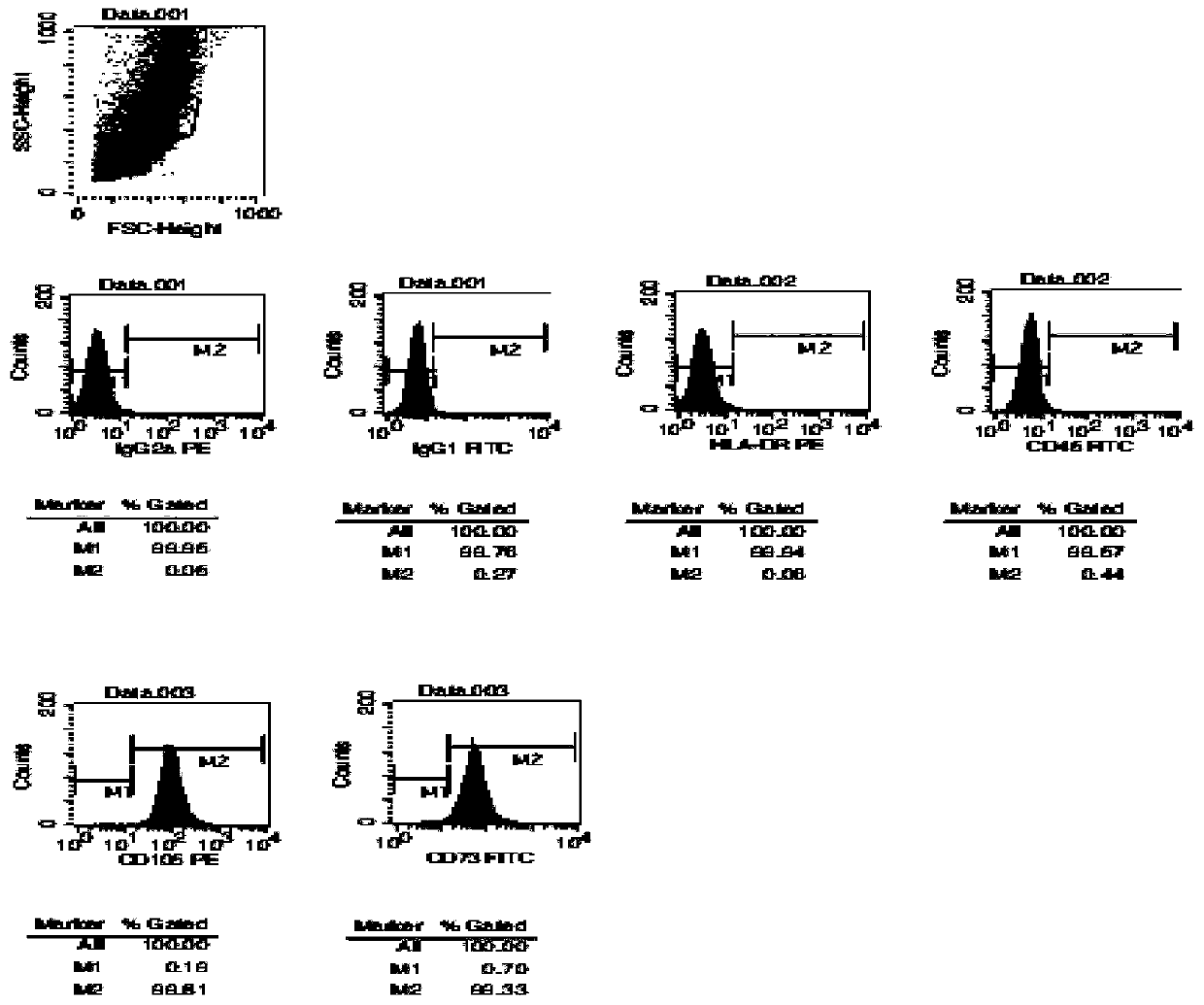
Patents
Literature
116 results about "Enzymic digestion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Broad-Spectrum Antibacterial and Antifungal Activity of Lactobacillus Johnsonii D115
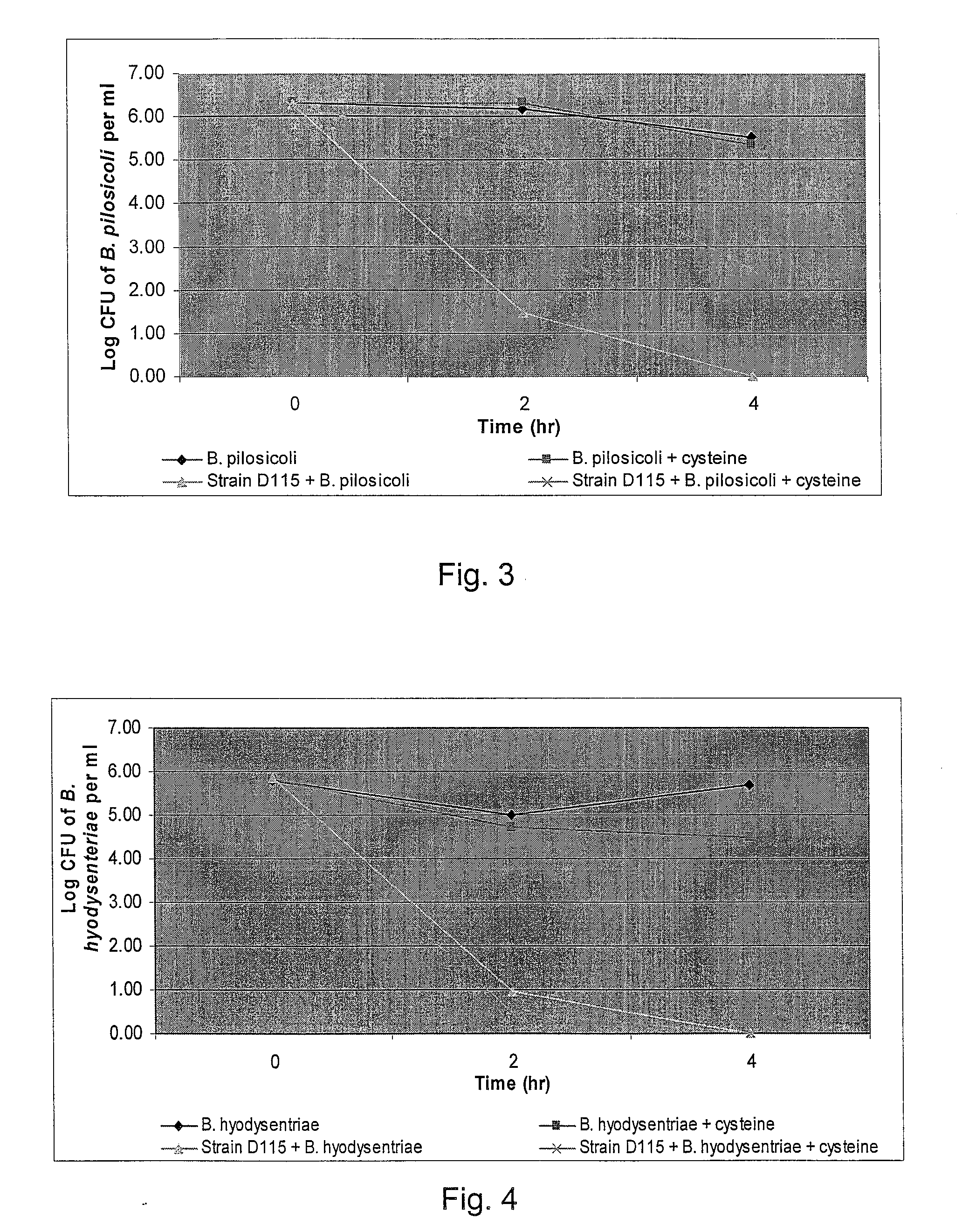
The present invention demonstrated the potential use of Lactobacillus johnsonii D115 as a probiotic, as a prophylactic agent or as a surface treatment of materials against human and animal pathogens such as Brachyspira pilosicoli, Brachyspira hyodysenteriae, Shigella sonnei, Vibrio cholera, Vibrio parahaemolyticus, Campylobacter jejuni, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, Clostridium perfringens, Yersinia enterocolitica, Escherichia coli, Klebbsiella pneumoniae, Staphylococcus aureus, Salmonella spp., Bacillus cereus, Aspergillus niger and Fusarium chlamydosporum. The proteineous antimicrobial compound was partially characterized and found to be heat tolerant up to 121° C. for 15 min, and acid tolerant up to pH1 for 30 min at 40° C. The compound is also stable to enzymatic digestion, being able to retain more than 60% antimicrobial activity when treated with pepsin and trypsin.
Owner:KEMIN IND INC
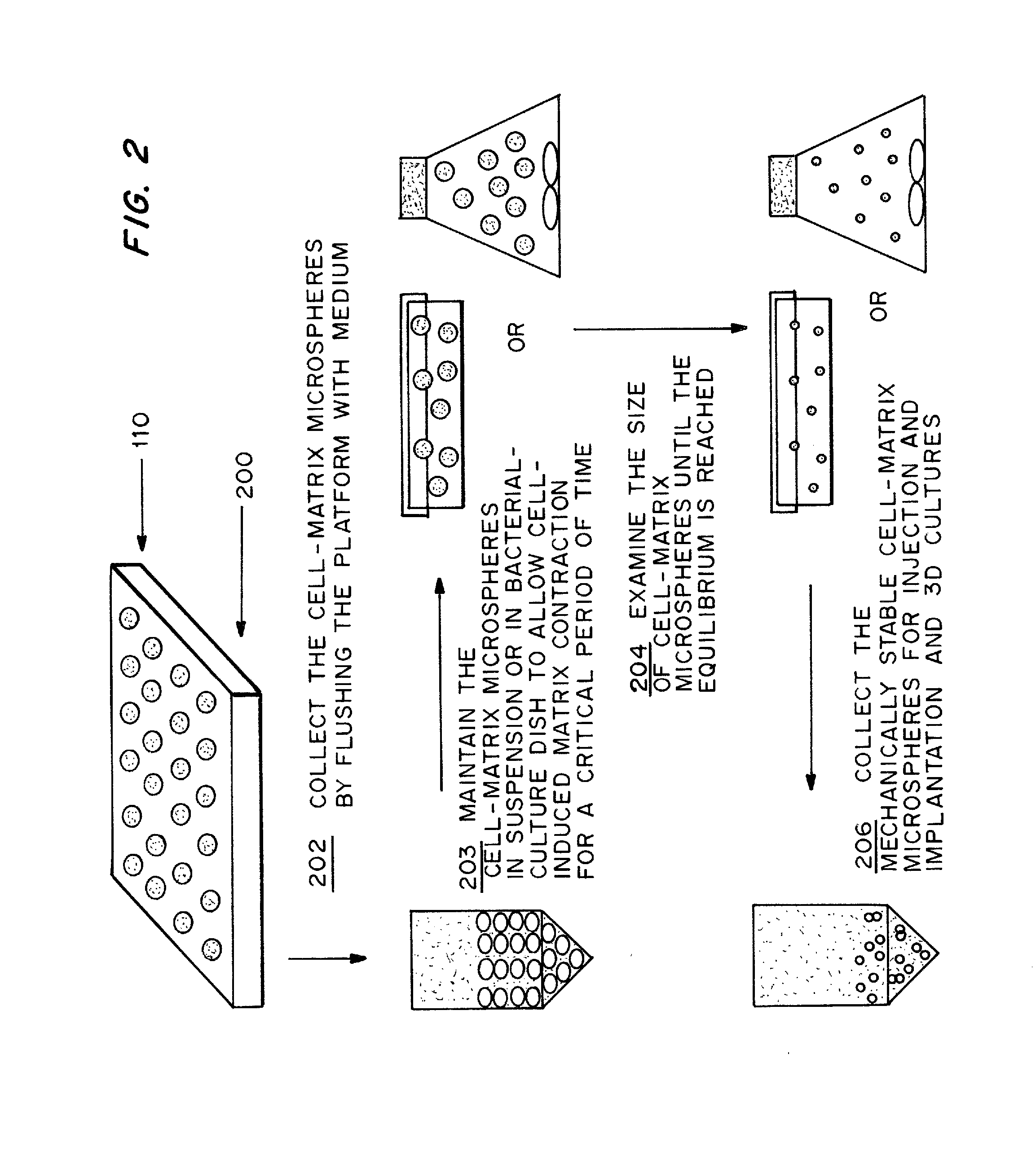
Cell-matrix microspheres, methods for preparation and applications
ActiveUS20080031858A1Increased protein productivityStable cell-matrix microspheresBiocideBioreactor/fermenter combinationsEnzymatic digestionHigh cell
A method has been developed to produce stable cell-matrix microspheres with up to 100% encapsulation efficiency and high cell viability, using matrix or biomaterial systems with poor shape and mechanical stability for applications including cell therapeutics via microinjection or surgical implantation, 3D culture for in vitro expansion without repeated cell splitting using enzymatic digestion or mechanical dissociation and for enhanced production of therapeutic biomolecules, and in vitro modeling for morphogenesis studies. The modified droplet generation method is simple and scalable and enables the production of cell-matrix microspheres when the matrix or biomaterial system used has low concentration, with slow phase transition, with poor shape and mechanical stability.
Owner:VERSITECH LTD
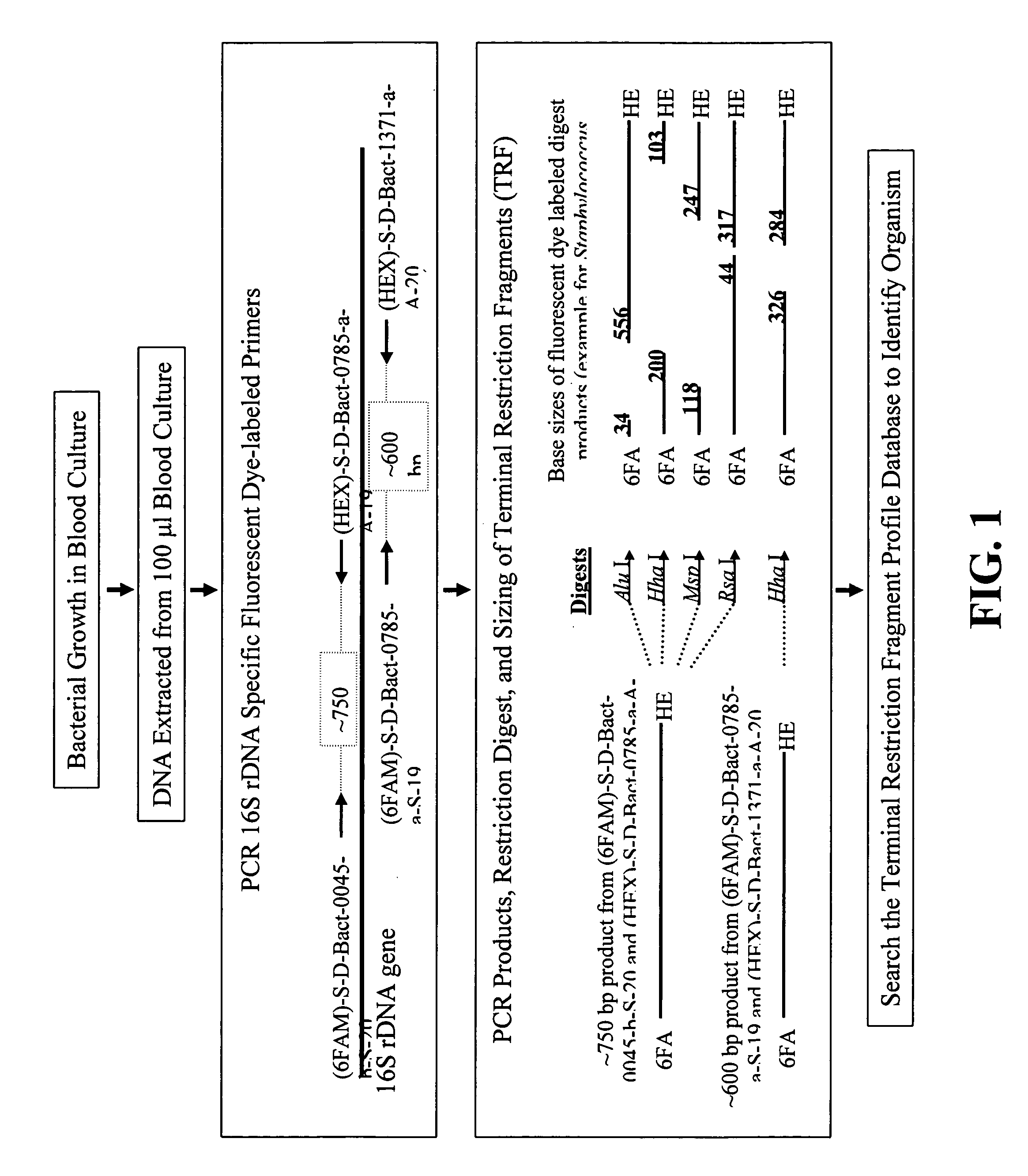
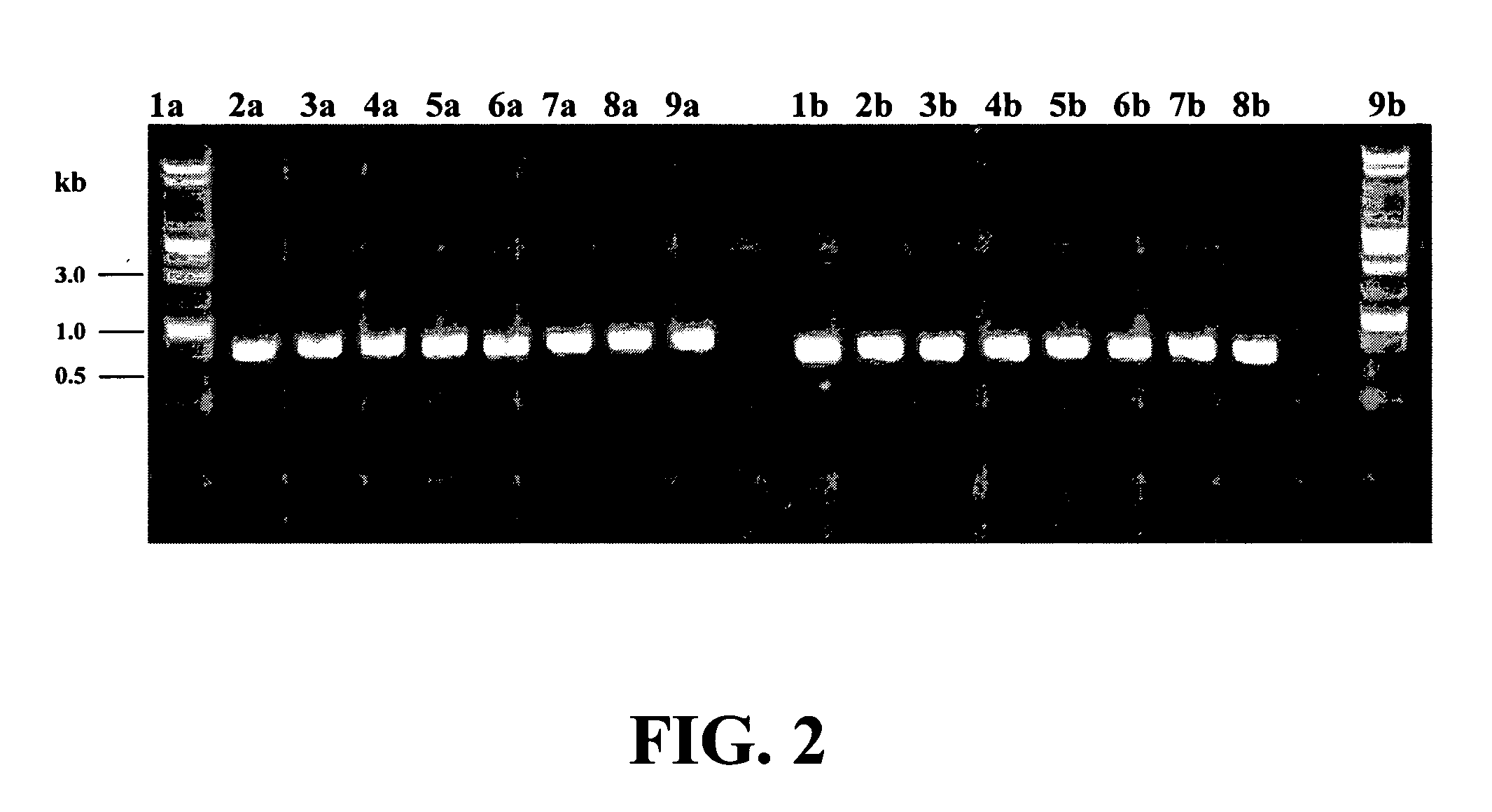
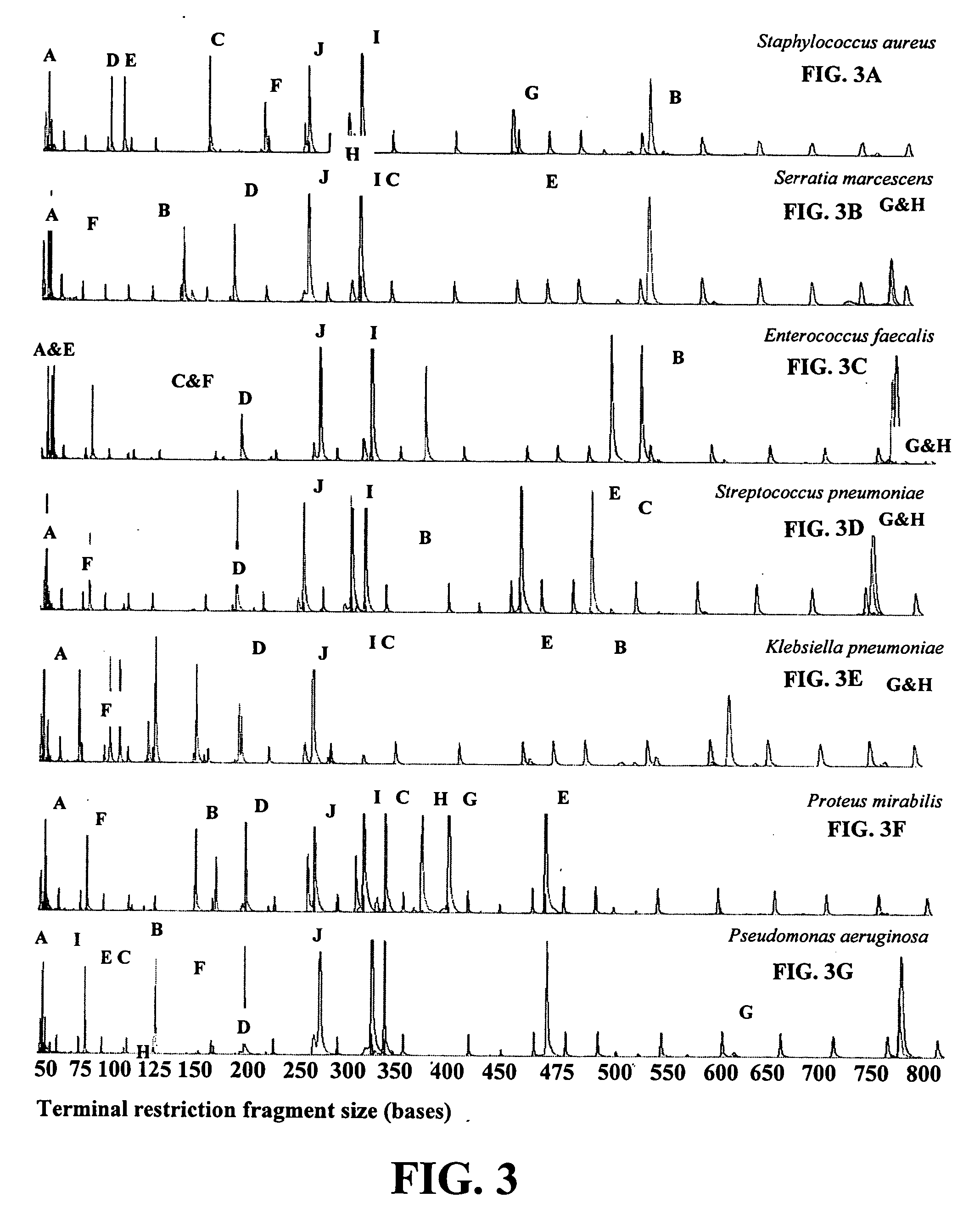
Rapid identification of bacteria from positive blood cultures
InactiveUS20050037408A1Reduce unnecessary useAvoid developmentMicrobiological testing/measurementBiological testingRapid identificationPositive blood culture
Disclosed is a method of detecting bacteria in a biological sample, especially a blood sample, without the need for extensive sub-culturing of the sample. Nucleic acid present within the sample is isolated and bacterial DNA specifically amplified using primers that uniquely prime the amplification of 16s rRNA-encoding nucleic acid. The amplicons are then digested with an endonuclease to yield a restriction fragment length profile for the biological sample. The restriction fragment length profile for the biological sample is then compare to a database of profiles made using cultures of known bacterial species. A match between the sample profile and the database quickly identifies the bacteria present in the sample.
Owner:MARSHFIELD CLINIC
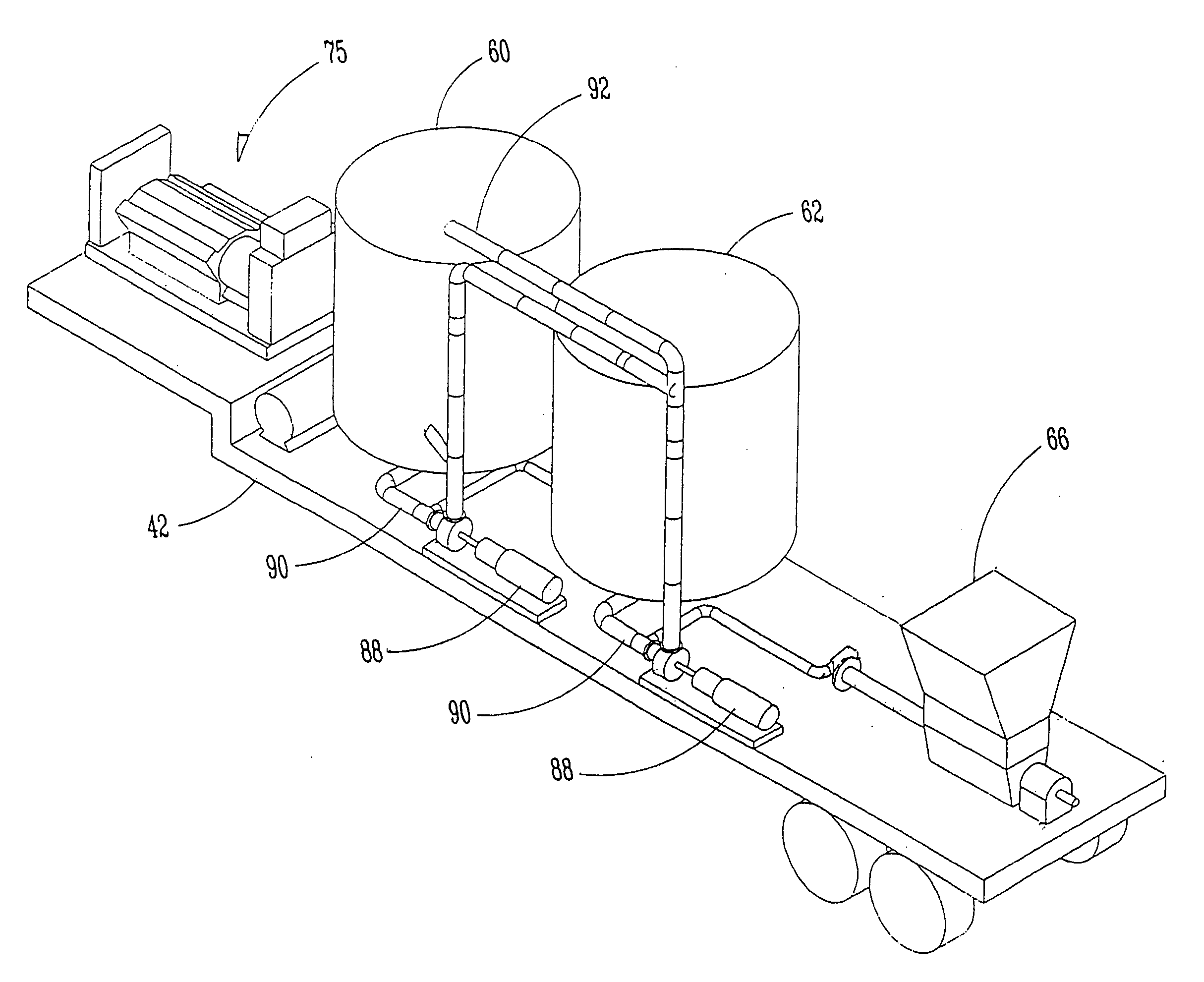
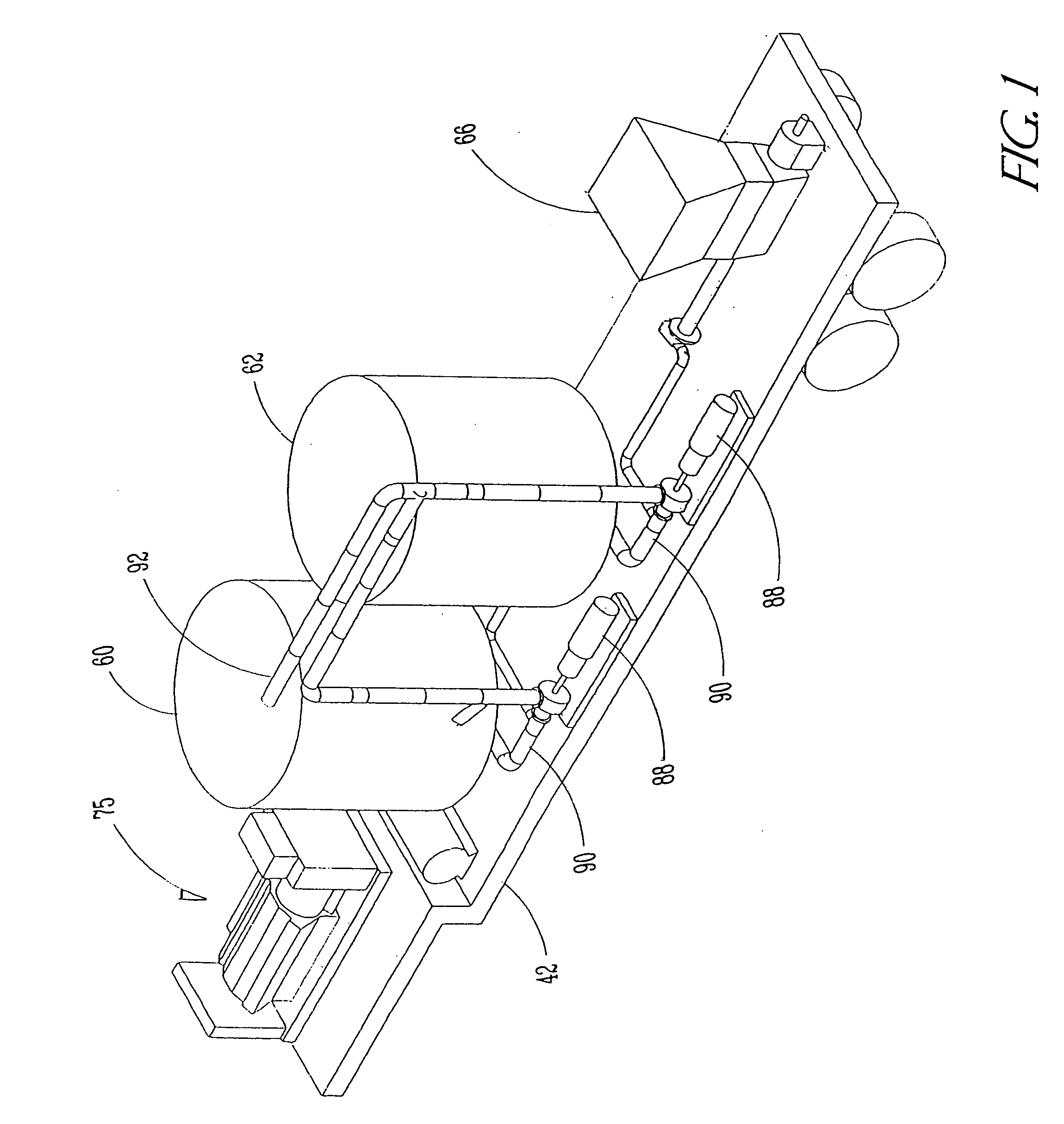
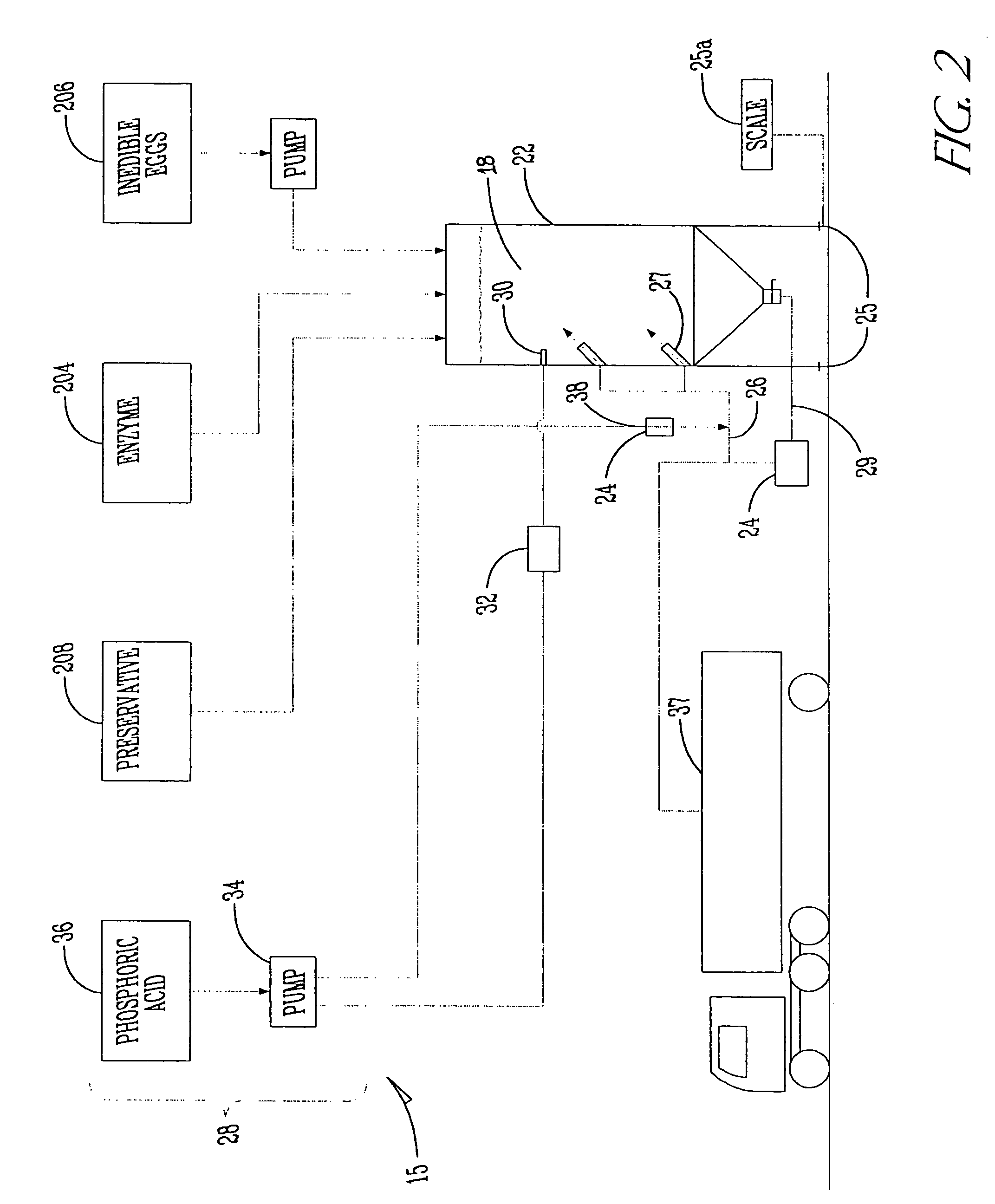
Apparatus for natural recycling of protein waste
ActiveUS20040265993A1Minimizing growth of bacteriaEfficient processBioreactor/fermenter combinationsSlaughtering animals fettering apparatusTruck-trailerProcessing plants
An apparatus and process for naturally recycling poultry carcasses for use as a nutritional supplement, the apparatus generally consists of four modules: an enzymatic digest medium mixing assembly that self adjusts for pH; a mobile grinding assembly mounted on a truck trailer; a digesting and emulsifying assembly which includes a heated tank and separator; and a drying system. Carcasses are loaded into the grinder, and the ground carcasses are pumped into a storage tank with the enzymatic digest medium to produce a protein soluble mixture. The particle size of this mixture is then further reduced, and transported to a centralized and stationary processing plant for digesting and emulsifying. The remaining emulsified proteins are then dried. The resulting pellet-like pieces are uniformly sized for packaging.
Owner:NATURALLY RECYCLED PROTEINS INC +1
Rapid preparation of nucleic acids by enzymatic digestion
InactiveUS20050164260A1Automate processRapid enzymatic digestionMicrobiological testing/measurementNucleic acid reductionCell cultureMolecular biology
The present invention provides methods, compositions and kits for isolating and purifying at least one nucleic acid directly from a biological sample, or for preparing a biological sample for subsequent isolation and purification of at least one nucleic acid, without removal of the biological sample's cell culture medium or cellular fluid.
Owner:SIGMA ALDRICH CO LLC
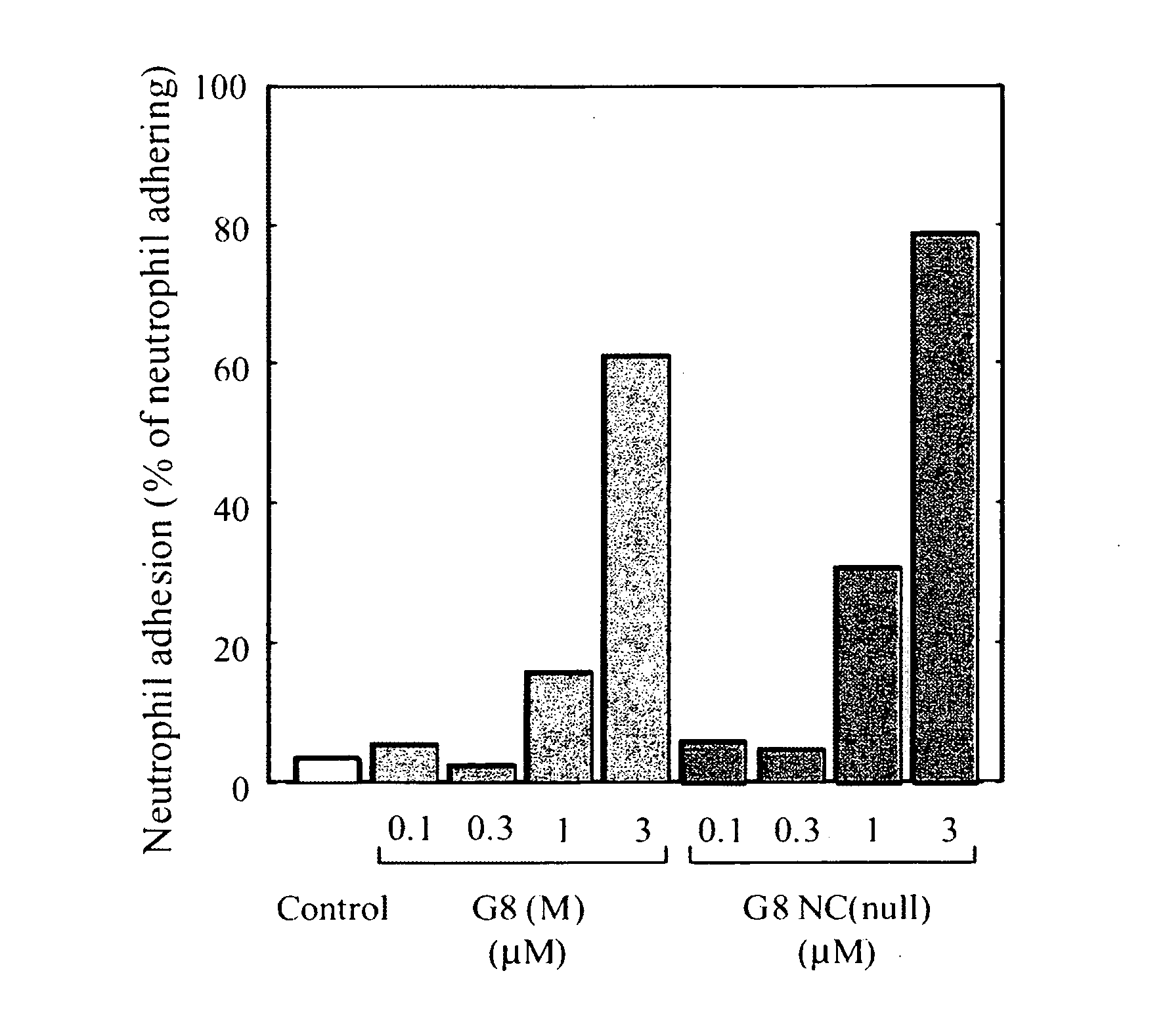
Novel Modified Galectin 8 Proteins and Use Thereof
InactiveUS20080044385A1More stabilityOrganic active ingredientsFungiEscherichia coliNeutrophil adhesion
Recombinant galectin 8 (rGal 8), produced in host Escherichia coli, exerts hemagglutinating activity, neutrophil adhesion inducing activity, integrin αM-binding activity, proMMP-9 binding activity, active form MMP-9 production promoting activity, superoxide production promoting activity, apoptosis inducing activity for a particular cell, suppressive or inhibitory activity against the metastasis / invasion of tumor cells, etc. In the rGal 8, however, a link domain linking two CRDs is highly susceptible to protease and, therefore, is very easily digestible with the enzyme, thereby losing the above activities. Thus, there is a need for a more stabilized molecule in view of further studies. Modification of the link domain linking two CRDs in galectin 8 provides a modified molecule having an elevated activity without any undesirable effects on the above activities.
Owner:GALPHARMA CO LTD
Placenta stem cell bank construction method and placenta tissue resuscitation method
InactiveCN104480533ALong storage timeKeep aliveEmbryonic cellsGerm cellsDrug biological activityResuscitation
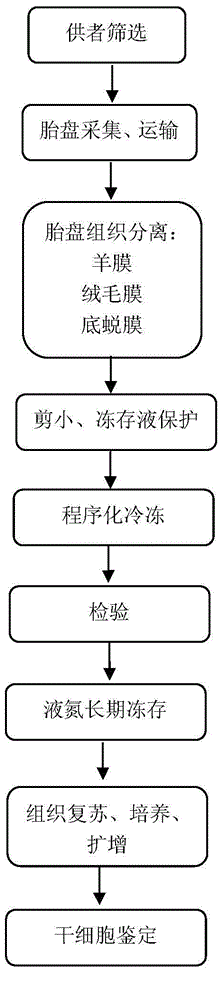
The invention discloses a placenta stem cell bank construction method and a placenta tissue resuscitation method, and relates to placenta stem cell bank construction methods. The placenta stem cell bank construction method comprises the following steps: after non-bacterial cleaning treatment to placenta tissues of at least three parts, the steps of tissue peeling, further sterilizing, shearing to be thin, protecting using a refrigerant, programmed freezing, cryogenic temperature temporary storage, long term storage with nitrogen canisters, and the like are carried out, and the placenta tissue with biological activities can be preserved for a long term. The placenta tissue preserved through the method can be used for the construction of a stem cell bank, resuscitation, enzymic digestion, cultivation, expansion, quality inspection and the like are performed after selective tissue part resuscitation according to required cell types, so as to obtain placenta amniotic membrance epithelial cells, placenta amniotic membrance mesenchyme, placenta chorion mesenchymal stem cell, placenta decidua serotina mesenchymal stem cells and the like. The method provided by the invention has the advantages that operation steps are simplified, manual intervention errors are reduced, the efficiency is improved, more stem cell resources are preserved, and a basis is provided for further personalized stem cell customization.
Owner:天晴干细胞股份有限公司
Decellularized porcine cornea tissue and preparation method and application thereof
The invention relates to the field of corneal stroma substitutions, and discloses a decellularized porcine cornea tissue and a preparation method thereof. The method comprises the steps: performing low-permeability swelling, repeated freeze thawing, enzymic digestion, ultrasonic treatment, sterilization and wet-state sealed storage in sequence on a corneal stroma sheet, which is cut under a germfree condition, of a fresh animal eyeball, wherein the enzymic digestion is implemented by a buffering solution containing DNA enzyme and RNA enzyme. The invention further provides the application of the decellularized porcine cornea tissue in serving as the corneal stroma substitution. According to the method, damage to the collagen structure of the corneal stroma is reduced to the maximum extent; collagen fibers are tidily arrayed, and the porosity is uniform and regular; no cell is retained; the lamellar structure is kept intact; the biocompatibility of a bracket material is improved; furthermore, on the premise that the corneal stroma is not modified, the decellularized porcine cornea tissue can be stored in a wet state for a long time.
Owner:QINGDAO CHUNGHAO TISSUE ENG
Method for separation and in-vitro culture of pancreatic cancer tissue organs of human
ActiveCN110317790AMaintain genetic heterogeneityImprove performanceCell dissociation methodsCulture processMatrigelDigestion
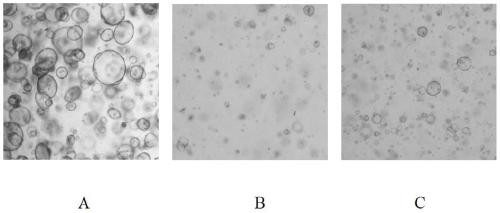
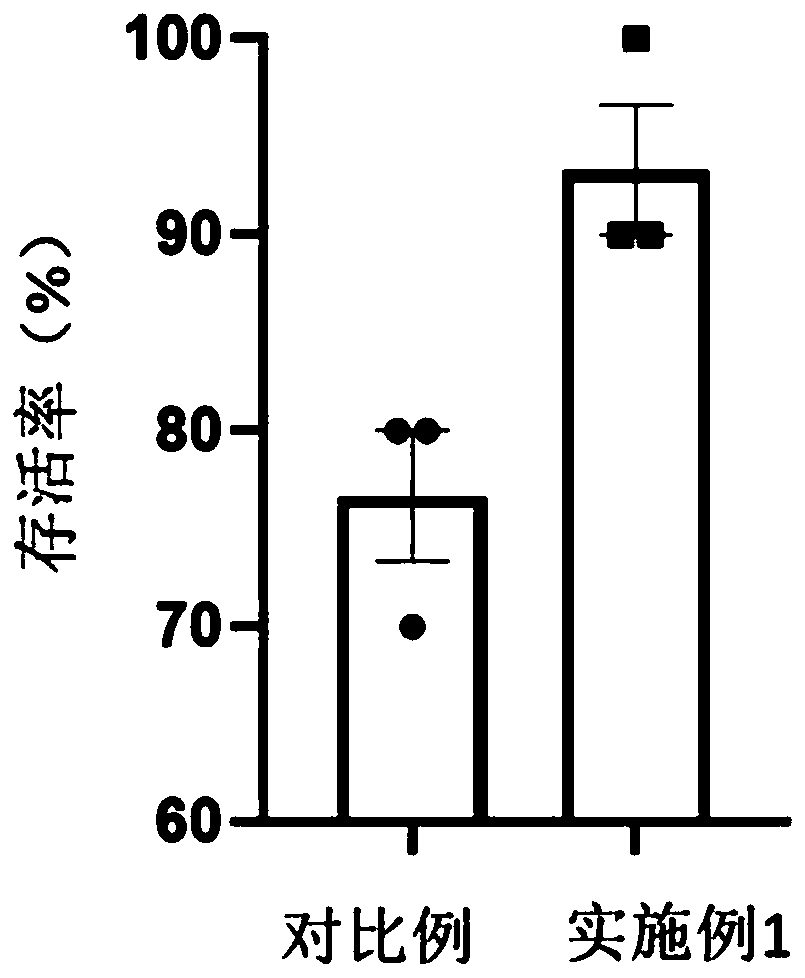
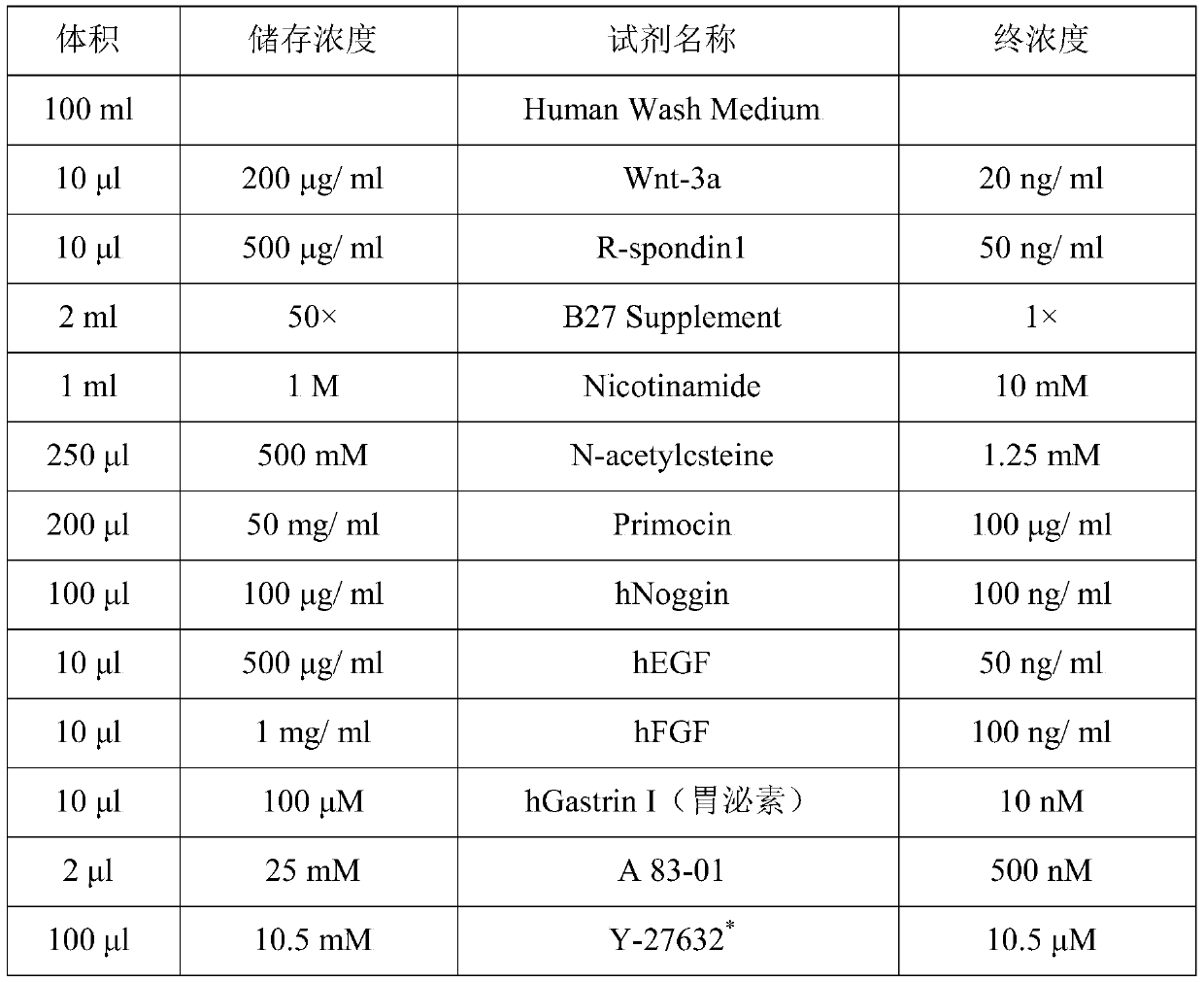
The invention discloses a method for separation and in-vitro culture of pancreatic cancer tissue organs of human. The method comprises the following steps that (1) after the pancreatic cancer tissue of human is cut, enzymic digestion is repeatedly carried out for 10-20 minutes at 37 DEG C in a rotation speed of 30-40 rpm three times, and a pancreatic cancer single cell is obtained; (2) the pancreatic cancer single cell and matrigel are uniformly mixed, then a culture medium containing a growth factor PEG2 and gastrin I are added for culture, and the pancreatic cancer tissue organs of human areobtained. The method for separation and in-vitro culture of the pancreatic cancer tissue organs of human has the advantages that the tumor tissue is digested by adopting a short-time repeated digestion method, which can effectively improve the cell yield and cell viability; the culture medium containing the growth factor PEG2 and the gastrin I is adopted for culture, which can effectively promotethe growth of the pancreatic cancer organs and increase the survival rate of the pancreatic cancer organs.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Method for establishing single cell transcriptome sequencing library and application of method
InactiveCN104389026AReduced amplificationIncrease the amount of effective dataMicrobiological testing/measurementLibrary creationSingle cell transcriptomeTranscriptome Sequencing
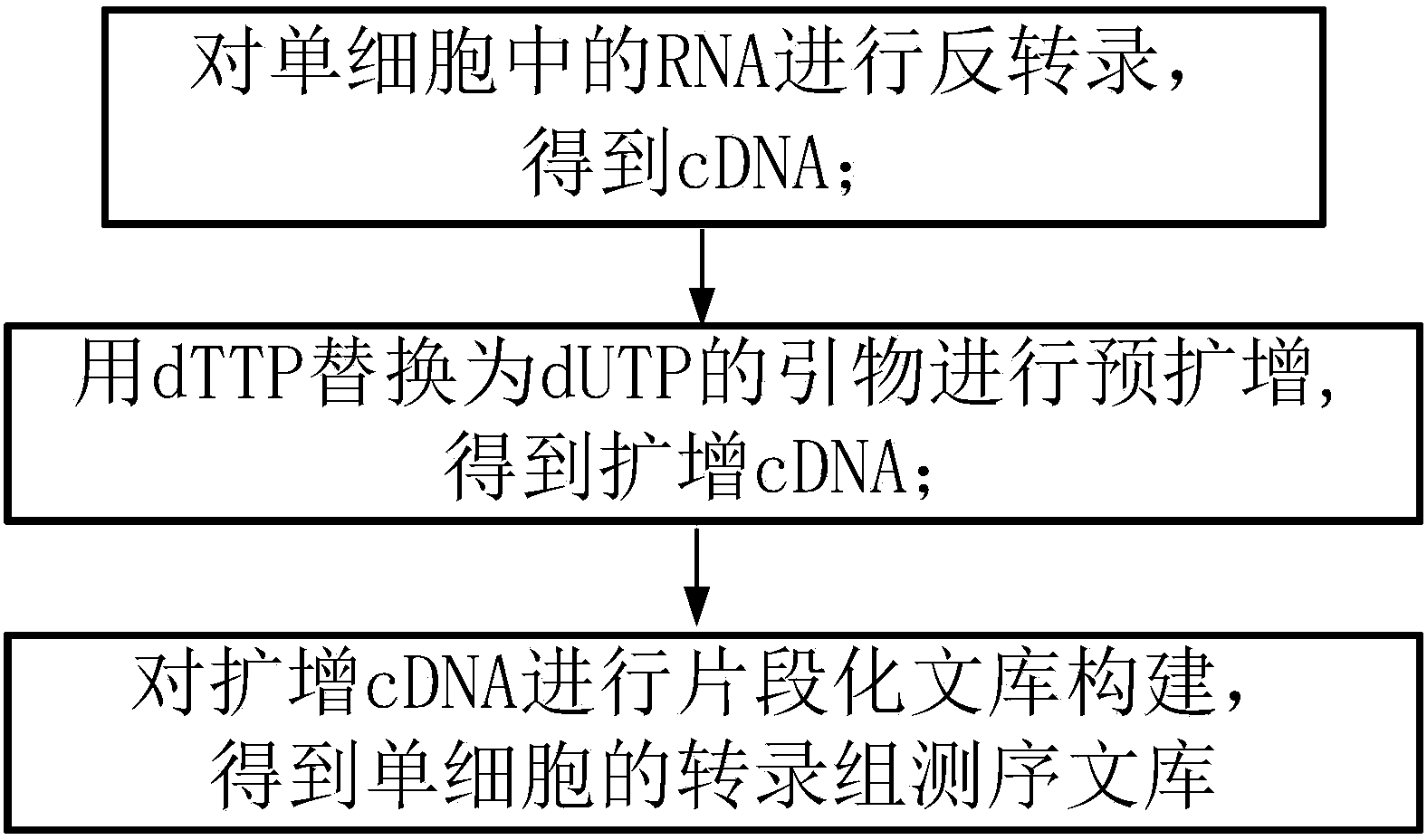
The invention discloses a method for establishing a single cell transcriptome sequencing library and application of the method. The method comprises the following steps: performing reverse transcription on RNA in a single cell, thus obtaining cDNA; performing pre-amplification on cDNA by using an amplification primer, thus obtaining amplified cDNA; and performing fragmentation library construction on the amplified cDNA, thus obtaining a transcriptome sequencing library of the single cell, wherein dTTP in the amplification primer is substituted by dUTP. According to the method disclosed by the invention, as the dTTP in the pre-amplification primer is substituted by dUTP, fragments containing the pre-amplification primer in a jointed fragment can be interrupted in an enzymic digestion step after the step of joint connection, and are removed in later high-temperature pre-denaturation and denaturation steps; furthermore, the amplification of fragments with the pre-amplification primer can be reduced, the ratio that the proportion of data with pre-amplification primer pollution in the obtained sequencing data is greatly reduced, and the effective data amount of the obtained data is greatly increased.
Owner:BEIJING NOVOGENE TECH CO LTD
Methylated DNA (Deoxyribonucleic Acid) detection method based on endonuclease digestion
ActiveCN102399861AFor mixed samplesSimple methodMicrobiological testing/measurementIndividualized treatmentDna amplification
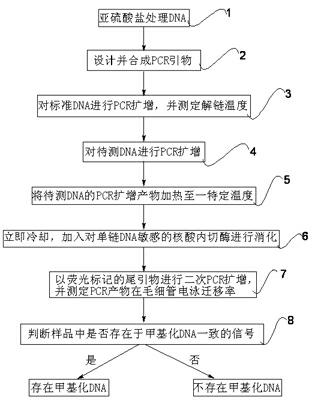
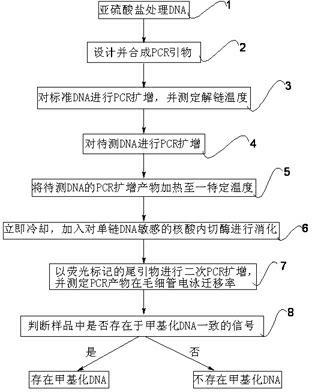
The invention relates to a methylated DNA (Deoxyribonucleic Acid) detection method which comprises the following steps of: (1) treating DNA to be detected, standard methylated DNA and non-methylated DNA by using sulfite; (2) synthesizing a PCR (Polymerase Chain Reaction) primer with a tailed primer sequence; (3) amplifying the standard methylated DNA and non-methylated DNA samples, and determining the melting temperature of the amplification product; (4) amplifying the DNA sample to be detected; (5) heating the DNA amplification product to be detected to a certain temperature; (6) digesting the DNA amplification product to be detected; (7) carrying out second PCR amplification by using a tailed sequence with a fluorescent mark; and (8) determining the electrophoretic mobility, and judging whether the sample contains methylated DNA. The invention has the advantages of large application scope, simple method and the like, the methylated DNA detection method requires small sample size, is not easy to be interfered and is applicable to mixed samples. The methylated DNA detection method has important significance in the fields of early detection, individualized treatment, condition judgment, relapse monitoring and the like for tumors.
Owner:江苏元丞生物科技有限公司
Method of selective peptide isolation for the identification and quantitative analysis of proteins in complex mixtures
InactiveUS7244411B2Efficient identificationWidely distributedIn-vivo radioactive preparationsComponent separationProtein insertionArginine
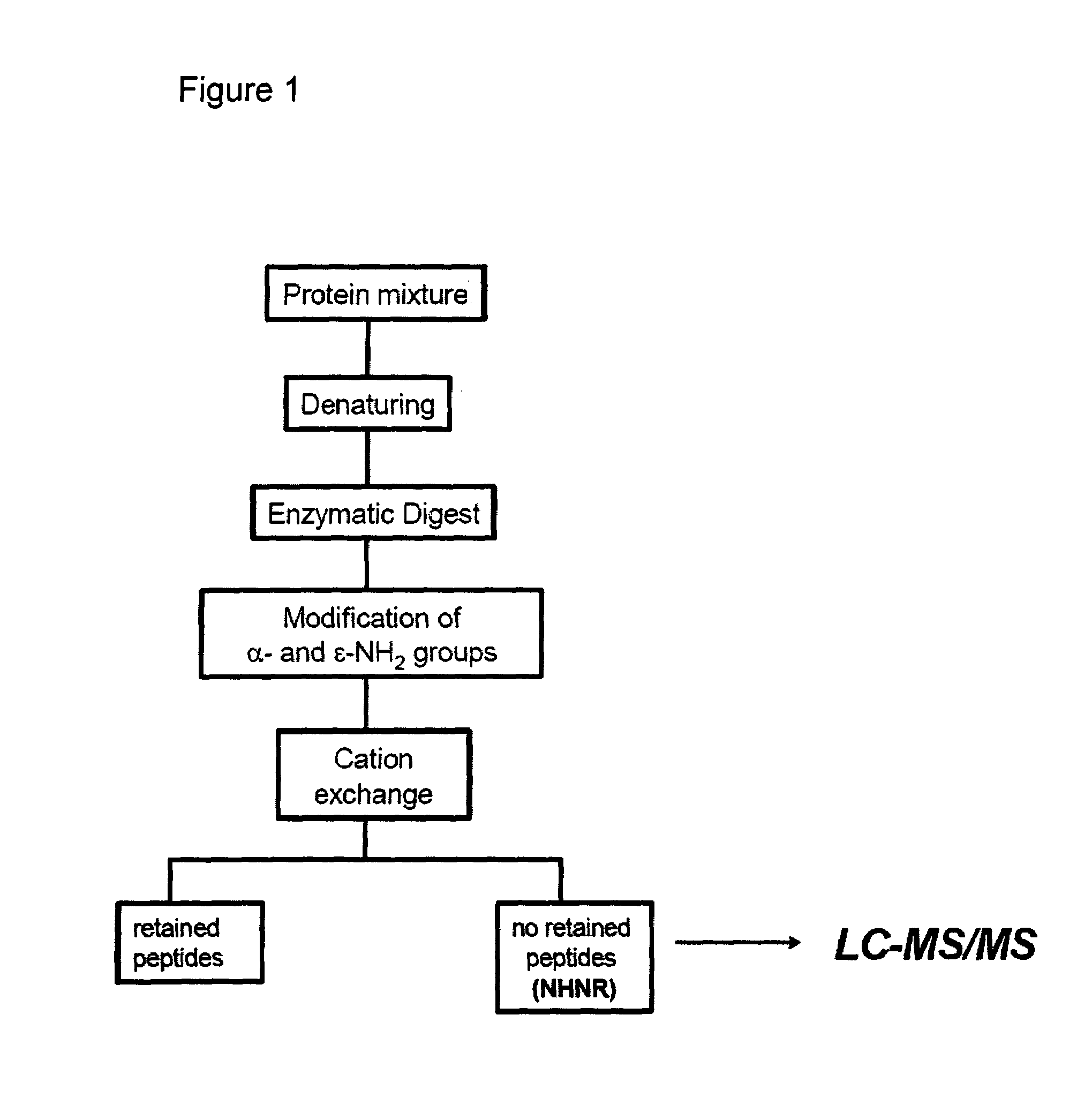
The present invention describes a method of selective peptide isolation for the identification and quantitative analysis of proteins in complex mixture. The method comprises the selective isolation from every protein of those peptides that neither contain arginine nor histidine (NHNR peptides), and the determination of the relative concentrations of one or several proteins in different samples from the ratio between the areas of the estimated theoretical spectra for the NHNR peptides labeled with different isotopes in each sample. The determination of the relative concentration of proteins is valid for any type of isotopic label of the NHNR peptides. The method avoids the separation and purification of the proteins present in a complex mixture, and the analysis of all peptides generated from the enzymatic digest of the samples. The method is applicable to the identification of proteins with vacunal, therapeutic and diagnostic aims.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Umbilical cord mesenchymal stem cell serum-free cultivation method and application
InactiveCN105602893ANo pollution in the processMaintain propertiesCell culture mediaCell culture supports/coatingDiseaseCuticle
The invention discloses an umbilical cord mesenchymal stem cell serum-free cultivation method, comprising the following steps: (1) preparing coating buffer for fibronectin, a recombinant human epidermal growth factor and a recombinant human fibroblast growth factor, and coating a plastic culture bottle; (2) taking human umbilical cord tissue Whartonps jelly, cutting into pieces, and adding into the culture bottle in the step (1); (3) for a product obtained in the step (2), adding into a serum-free culture medium; (4) putting into a 5-percent carbon dioxide incubator at 37 DEG C for culture, wherein mesenchymal stem cells overgrow in 10-12 days; (5) washing by a phosphate buffer solution, and performing Triple enzymic digestion; (6) adding the phosphate buffer solution, and stopping the digestion; (7) collecting mesenchymal stem cell supernate; (8) centrifuging; (9) collecting sediments to obtain the needed mesenchymal stem cells. By adopting the method, the mesenchymal stem cells can go down to the 20th generation, the characteristics of the mesenchymal stem cells are kept, diseases can be treated, and application prospect is achieved.
Owner:SHANGHAI JIQUAN BIOTECH CO LTD
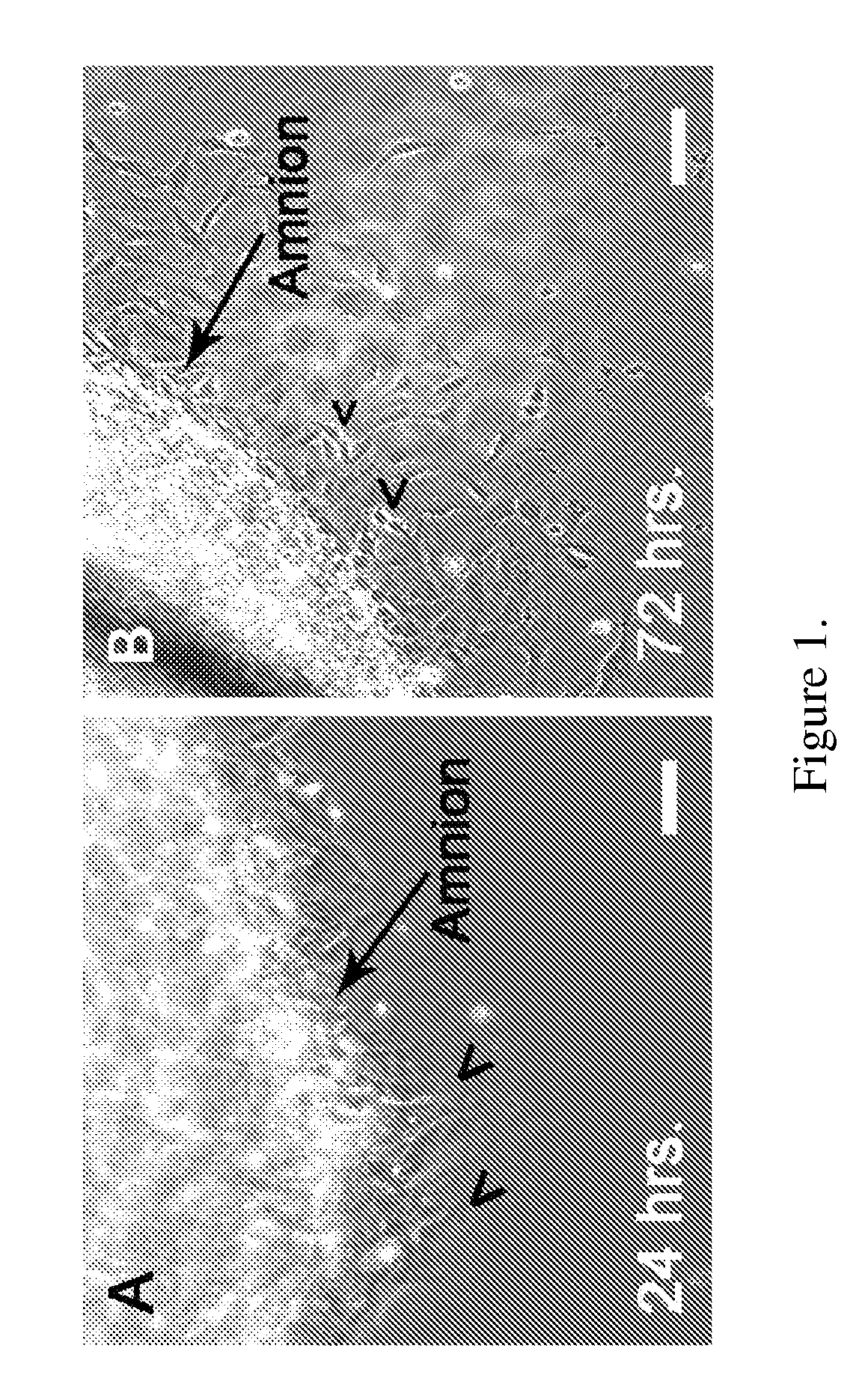
Obtaining multipotent amnion-derived stem cell (ADSC) from amniotic membrane tissue without enzymatic digestion
Owner:RUTGERS THE STATE UNIV
Separation method and culture in vitro method for mouse female germline stem cells
InactiveCN102382798ASimple methodLow requirements for experimental conditionsGerm cellsMagnetic beadStem cell culture
The invention provides a separation method for mouse female germline stem cells in the technical field of transgenosis engineering. The separation method comprises the following steps: step one, collecting a mouse ovary, and adopting a two-step enzymic digestion method for preparing germline stem cell suspension; and step two, adding mvh first antibody and then second antibody magnetic bead in the cell suspension and re-suspending the cells, thus separating the female germline stem cells. The invention also provides a culture in vitro method for the mouse female germline stem cells, which comprises the following steps: transferring the female germline cells on an STO cell culturing layer, adding female germline stem cell culture solution, and conducting primary culture and subculture on the female germline stem cells, thus obtaining purified and stable germline stem cells. In the method, the mouse female fermline stem cells are separated and cultured in vitro successfully at first time, the methods are simple, the cost is low, the requirements on experiment conditions are low, and the operability is strong.
Owner:吴际
Detection of transcription factor protein by double chain RNA molecule with special labeling fixed to micro-plate coated by exonuclease III
InactiveCN1721547ALimit commercializationHigh Throughput Analysis CapabilitiesMicrobiological testing/measurementRegulation of gene expressionTissue extracts
Transcription factor is one important protein for gene regulation, is center of gene expression regulating passage and network, is important target of functional genome and protein block research and important target site of transcription treatment and medicine research. The present invention proposes exonuclease III digesting double stranded nucleic acid molecule coated in microporous plate and containing special marker to detect transcription factor protein. The process of analyzing transcription factor expression and activation level includes the following steps: preparing nucleic acid; fixing the nucleic acid molecules to the pores of the microporous plate; incubation of cell or tissue extract containing transcription factor in the plate; incubation of exonuclease III reaction liquid in the plate; incubation of specific conjugate with special marker on nucleic acid on the plate; detecting and analyzing the specific conjugate to obtain the expression and activation level.
Owner:王进科 +1
Method for continuous and batch purification of bacterial magnetic particles
InactiveCN101372364AResidue reductionContinuous operationFerroso-ferric oxidesIron sulfidesHigh pressureMagnetotactic bacteria
The invention provides a method for purifying a bacterial magnetosome at a large scale, which comprises the following steps: breaking magnetotactic bacterial cells by a high-pressure homogenizer, separating and cleaning the magnetosome by a magnetic chromatography system, digesting membrane protein with protease k, electroeluting, desalting by a magnetic stirring system, etc. The method has the advantages of continuous operations, and that the purification period is shortened from 2-3 months to less than one week, damage caused by ultrasonication to the plasma membrane of the magnetosome is effectively reduced, the membrane protein on the surface of the magnetosome is reduced and the immunogenicity of the purified magnetosome is lowered.
Owner:CHINA AGRI UNIV
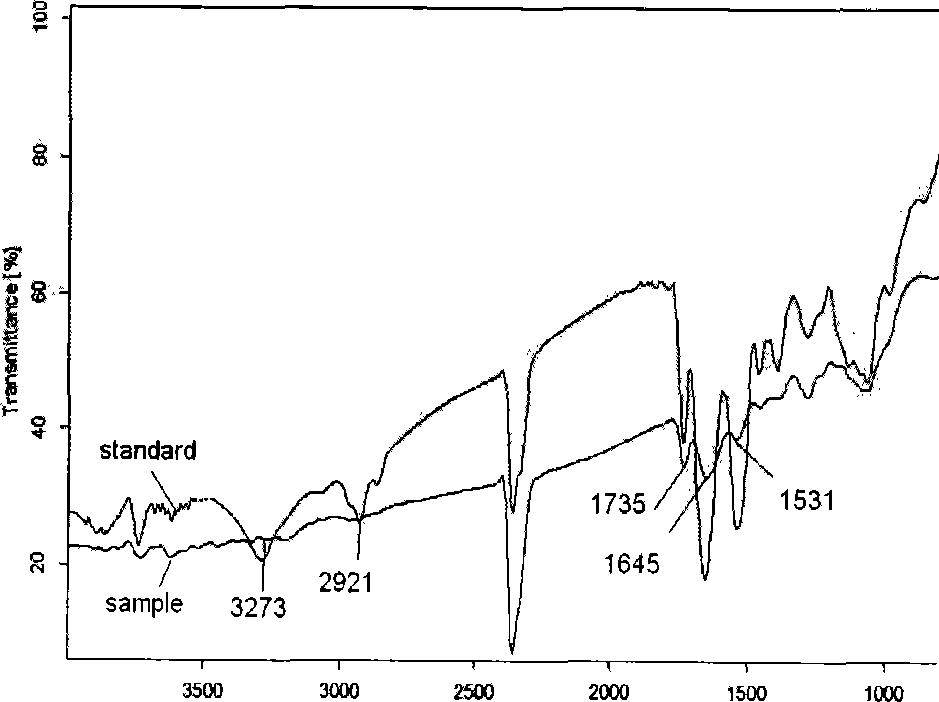
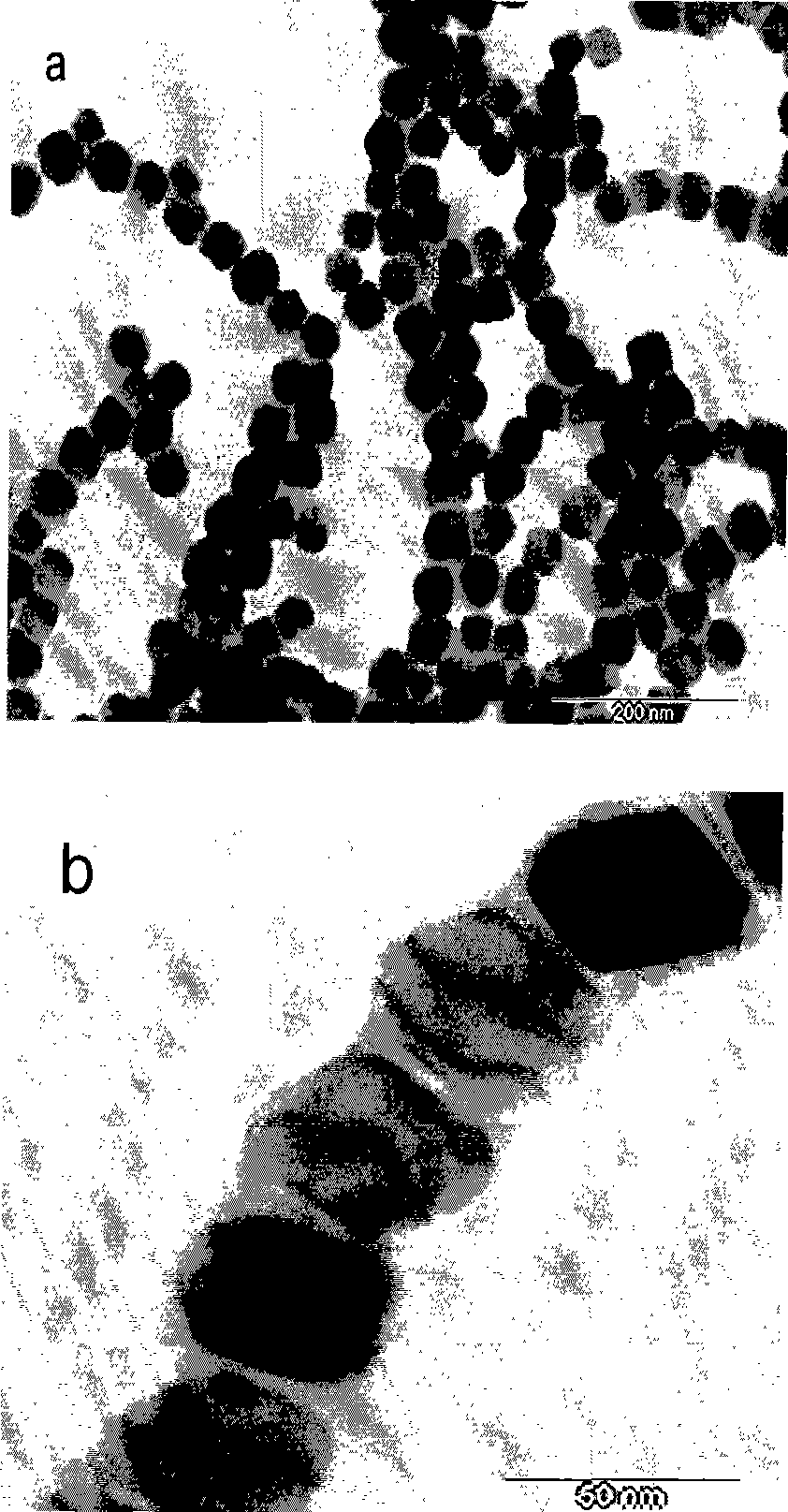
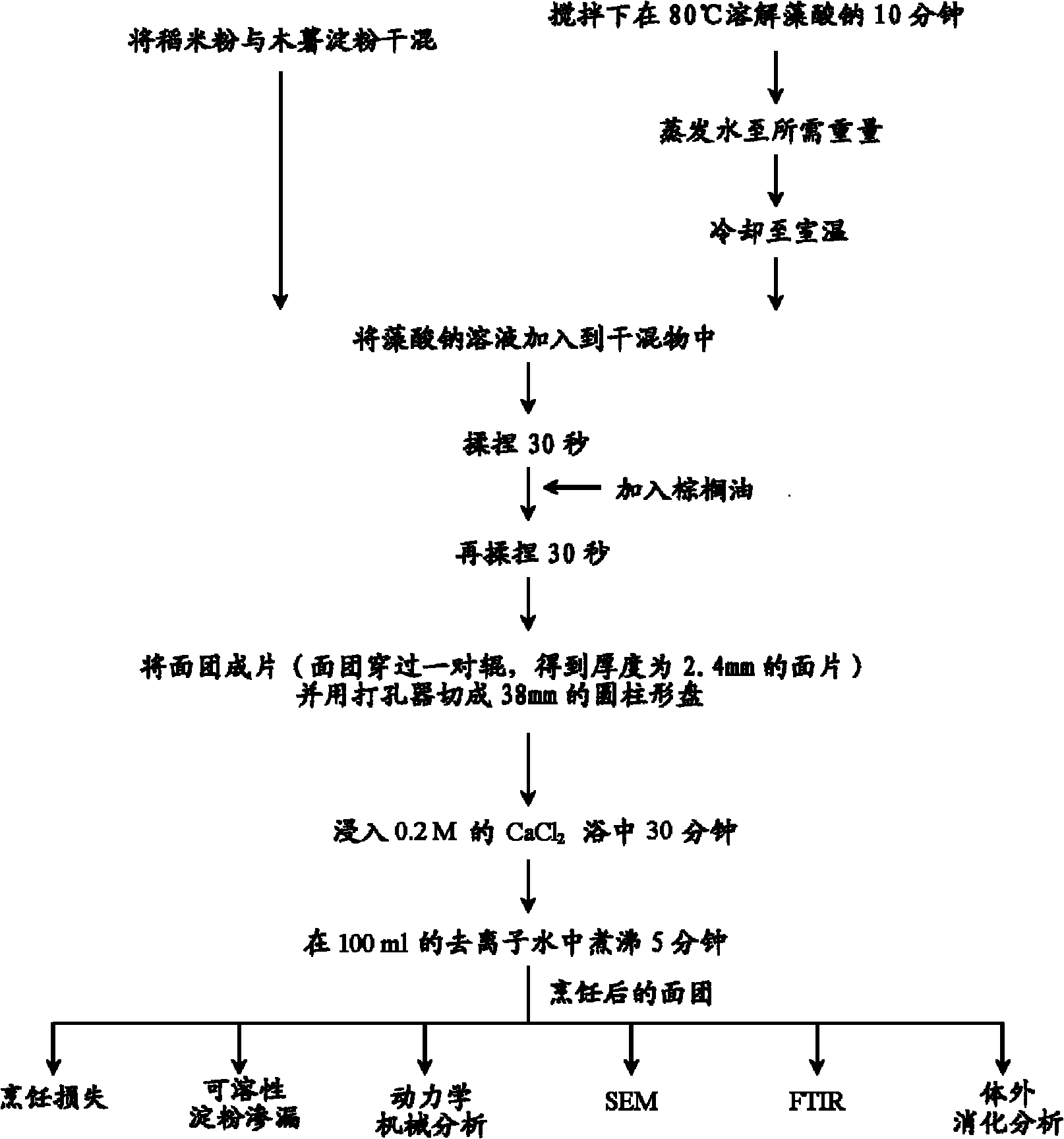
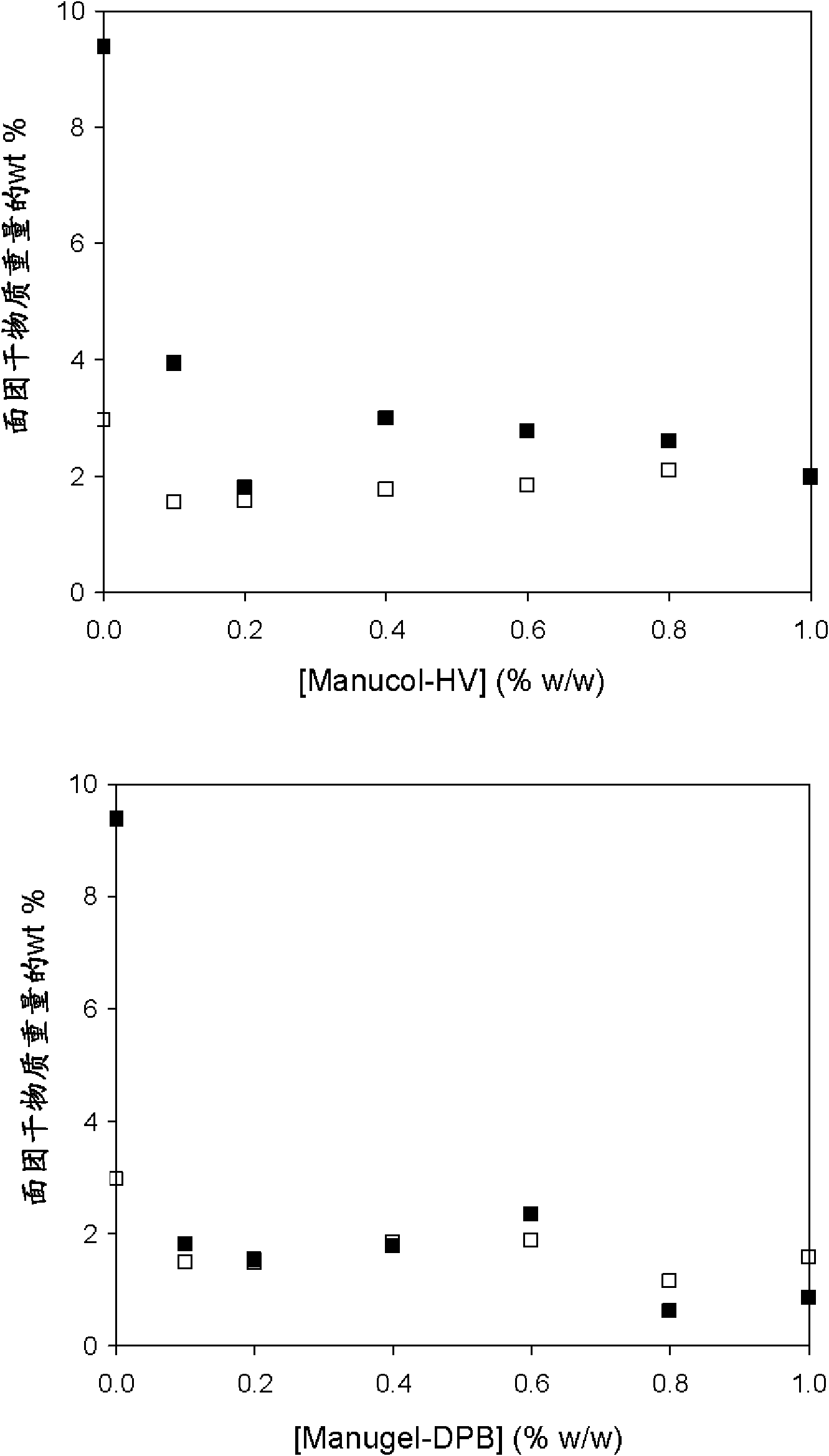
Method of reducing enzymatic digestion rates of starch granules in food and food products produced therefrom
The present invention describes a method of reducing the enzymatic digestion rates of starch granules in food, particularly rice-based food. The method is carried out by encapsulating the starch granules with a reaction compound formed by the chemical reaction of at least a crosslinkable polysaccharide that has been pre-mixed with the ingredients for food production, and at least a crosslinking agent. The invention also relates to a process of preparing food products by incorporating the method of the present invention and food products produced by the present method.
Owner:NESTEC SA
Method for extracting and purifying eimeria tenella merozoite
ActiveCN102796669AEasy to operateLow costProtozoaMicroorganism based processesEmoia loyaltiensisEimeria
The invention discloses a method for extracting and purifying eimeria tenella merozoite. The method comprises the steps of: releasing the merozoite and purifying the merozoite, wherein the merozoite release comprises mechanical grinding release and enzymic digestion release, 0.0625% pancreatin and 0.175% sodium taurodeoxylate are adopted as digestive juice in the enzymic digestion release process; digestion is moderate, which is beneficial to the activation of the merozoite, can eliminate erythrocytes to obtain pure merozoite. The method is convenient and simple to operate, is low in cost, and is ideal in separation and purification effects; the separated merozoites are large in quantity, are pure with few impurities and keep vigorous energy; and the method is suitable for research on drug susceptibility detection, vaccine research and other biological researches.
Owner:GUANGXI VETERINARY RES INST
Human pulmonary artery smooth muscle cell separation and culturing method and application of same
InactiveCN102168062AConducive to survivalAvoid damageArtificial cell constructsVertebrate cellsDiseaseCell survival
The invention relates to a method of separating and culturing human pulmonary artery smooth muscle cells from strips of PTE and the cells' application in studies of diseases related to lung circulation. The method comprises treating cell membranes by single enzymic digestion method to decrease damage degree of the cells, thereby benefiting survival of the cells. The culturing method has stable technology and good repeatability, the cultured cells have uniform forms, and the growth of the cells is good. At the same time, the cells obtained provide rich material source with strong singularity for further study on lung circulation diseases, particularly for experiments on Chronic Thromboembolic Pulmonary Hypertension patients' pathogenesis, diseases generation and development processes and PTE prognosis.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV +1
Method for preparing umbilical cord mesenchymal stem cells
InactiveCN104988118AImprove qualityHigh activitySkeletal/connective tissue cellsUmbilical cord tissueHyaluronidase
The invention provides a method for preparing umbilical cord mesenchymal stem cells and aims to obtain umbilical cord tissues. The method comprises the steps of conducting digestive treatment on the umbilical cord tissues with Whartons jelly digestive juice, and collecting the digestive juice, wherein the Whartons jelly digestive juice comprises hyaluronidase and a chondroitin sulfate ABC enzyme which separate and extract the umbilical cord mesenchymal stem cells from the digestive juice. By means of the method for preparing the umbilical cord mesenchymal stem cells, the hyaluronidase and the chondroitin sulfate ABC enzyme are adopted for digesting Whartons jelly in a joint mode in the enzymic digestion process, the umbilical cord mesenchymal stem cells with the best cell quality can be obtained on the whole, the influence of long-time digestion on cell bodies by a pancreatic enzyme and collagenase is avoided, and the obtaining rate of the umbilical cord mesenchymal stem cells which are finally obtained through the preparation is higher; the obtained stem cells have higher cell viability and differentiation potential.
Owner:SHEN ZHEN ISTEM REGENERATIVE MEDICINE SCI TECH CO LTD
Method for constructing Kareius bicoloratus liver cell line
InactiveCN102321569AAvoid influencePromote rapid proliferationArtificial cell constructsVertebrate cellsMedicinal herbsDisease
The invention relates to a method for constructing a Kareius bicoloratus liver cell line. The method comprises the following steps of: starting primary culture by a two-step enzymatic digestion method by taking liver tissue cells of Kareius bicoloratus as a material, and culturing in a dulbecco's modified eagle medium (DMEM) / F12 culture solution which contains fetal calf serum, liver cell growth factor, chondroitin sulfate, N-acetyl glucose hydrochloride and Kareius bicoloratus liver extracting solution and has a pH value of between 7.0 and 7.4; and subculturing by a trypsin digestion method.The process is scientific and reasonable, the damage of an enzymatic digestion method to liver cells of the Kareius bicoloratus in the construction of the conventional cell line is avoided, the cellscan normally adhere to walls to grow, various culture solution additives effectively promote the proliferation of the liver cells, the Kareius bicoloratus liver cell line constructed by the method ispassed to 110th generation at present, the proliferation state of the cells is good, and the cell line is expected to be applied to the research on the antiviral action mechanism of active ingredients of a plurality of kinds of Chinese medicinal herbs on fish viruses at molecular and cellular levels, so that an effective therapeutic medicine or an immunopotentiator in disease control is developed, and various aquaculture diseases which become increasingly serious are solved.
Owner:SHANDONG ANALYSIS & TEST CENT
Exonuclease III digesting FRET-dsDNA microarray chip for detecting transcription factor protein
InactiveCN1733934ASolve application problemsEnable high-throughput detectionMicrobiological testing/measurementBiological testingProtein detectionFluorescence
Disclosed is a transcription factor protein detection method through exonuclease III digestion FRET-dsDNA micro array chips, wherein the expression and activation levels of the transcription factor are analyzed through the following steps: (1) preparing FRET-dsDNA micro array chips, (2) reacting the transcription factor with FRET-dsDNA micro array chips, (2) reacting the exonuclease III with FRET-dsDNA micro array chips, (1) proceeding detection and analysis to the FRET-dsDNA micro array chips.
Owner:王进科 +1
Method for extracting mesenchymal stem cells from umbilical cord Wharton's jelly
The invention belongs to the technical field of bioengineering, and in particular relates to a method for extracting mesenchymal stem cells from umbilical cord Wharton's jelly. The method comprises the steps of separating the Wharton's jelly, digesting the Wharton's jelly, centrifugally separating liquid after being digested, processing separated liquid at low temperature, centrifugally separating liquid after being processed at low temperature, culturing and the like. The method for extracting the mesenchymal stem cells from the umbilical cord Wharton's jelly provided by the invention is a method for obtaining mesenchymal stem cells of an umbilical cord with high efficiency and high purity; the yield and the purity of the mesenchymal stem cells of the umbilical cord are increased by utilizing enzymic digestion in combination with gelatin spreading; the separation and obtaining time is effectively shortened; a method for rapidly preparing and identifying is realized; and the yield, which is higher than the yield in the previous culture method, can be obtained.
Owner:HUBEI SHENGMINGYUAN STEM CELL CO LTD
16S rDNA based preparation method of bacteria nucleic acid fingerprint characteristic spectrums and application thereof
InactiveCN103060431AValid identificationUndisturbedMicrobiological testing/measurementMicroorganism based processesEnzymatic digestionSuper absorbent
The invention discloses a method for preparing bacteria 16S rDNA fingerprint spectrums. The method comprises the steps of PCR (polymerase chain reaction) amplification, SAP (super absorbent polymer) enzymatic digestion, transcription and nuclease digestion, purification, mass spectrometer detection and the like. A nucleic acid fingerprint spectrum database of common bacteria is created based on the method. According to the mass spectrum peak chart generated through experiments, the bacteria to be detected can be classified and identified and the results can be widely applied to the fields of bacteria classification and identification, genetic evolution analysis, drug resistance screening application, import and export inspection and the like.
Owner:向华
Human autologous adipose mesenchymal stem cell serum-free culture method
InactiveCN110846275AExcellent adhesionPromote proliferationCulture processSkeletal/connective tissue cellsSerum freeMesenchymal stem cell
The invention relates to a human autologous adipose mesenchymal stem cell serum-free culture method which particularly includes the steps: selecting sterile adipose tissues and performing first digestion to obtain first cell suspension; performing second digestion on undigested adipose tissues to obtain second cell suspension; combining the first cell suspension and the second cell suspension andthen performing culture, passage and identification to obtain human autologous adipose mesenchymal stem cells. According to the adipose mesenchymal stem cell culture method, primary cell separation, multiplication culture and cryopreservation systems are serum-free culture media, and the risk of animal derived ingredient addition is completely avoided. The method is a two-step enzymic digestion method, by the aid of the specific proportion of digestive enzyme and the adipose tissues, the adipose tissues sufficiently contact with enzyme solution and can be sufficiently digested, the cells cannot be damaged, primary cells are high in yield, good in activity, high in repeatability and good in safety, and the method meets clinical application standards.
Owner:赛瑞诺(北京)生物科技有限公司
Sterile immunogenic non-tumorigenic tumor cell compositions and methods
InactiveCN1774255AStay aliveMaintain metabolic activityMammal material medical ingredientsCancer antigen ingredientsCell AggregationsMetastasis tumor
This invention relates to methods of removing bioburden from an aggregate of cells to obtain sterile cells that remain viable and immunogenic for the production of vaccines. This invention further relates to a method of eliciting an immune response to prevent a recurrence of metastases that involves preparing and administering a sterile vaccine derived from solid tumors. The vaccine is prepared by excising a solid tumor from a cancer patient, digesting the tumor cells with an enzyme to obtain dissociated cells, irradiating the dissociated cells to render the cells non-tumorigenic, and sterilizing the cells.
Owner:INTRACEL RESOURCES
Recovery processing method for increasing reuse times of ultra-microcarrier
ActiveCN102586173AIncrease usageEasy to handleArtificial cell constructsVertebrate cellsMicrocarrierComputer science
The invention relates to a recovery processing method for a microcarrier, in particular to a recovery processing method for increasing reuse times of an ultra-microcarrier, which includes steps of screening, diluting a solution, stirring, filtering, processing by using sodium hydroxide, adjusting pH value, processing of enzymic digestion, washing, processing of sterilizing and the like. The recovery processing method for increasing reuse times of the ultra-microcarrier adopts combination of a plurality of steps to achieve a best processing effect, remarkably increases use times of the microcarrier, and saves use cost. The microcarrier after being processed for a plurality of times does not have remarkable effect on wall-attaching growing conditions in the microcarrier of a cell during use. The processing method can be widely applied to recovery of various microcarriers.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +1
Human placenta mesenchymal stem cell, preparation method and application thereof
ActiveCN110079498AHigh purityHigh activityCell dissociation methodsSkeletal/connective tissue cellsAntigenAdipogenesis
The invention provides a human placenta mesenchymal stem cell, a preparation method and an application thereof. The preparation method comprises the following steps: collecting, separating, culturing,cryopreserving, detecting, recovering, and the like. A high-purity high-activity mesenchymal stem cell can be acquired by only performing slide adherent culture after a human placenta chorion tissueblock is acquired in the separating process; the technological process is simplified; the cost of enzymic digestion is saved; the adherence and growth rate of primary cells are accelerated; the cell culture period is shortened; the introduction of more external interference factors is avoided, so that the process stability can be easily controlled. The multiplication capacity of the human placentamesenchymal stem cell acquired according to the invention is more stable than that of other mesenchymal stem cells; after the human placenta mesenchymal stem cell passes to P20 generation, the cell still can stably proliferate, and the cellular morphology, molecular surface antigen and adipogenesis osteogenesis differentiative potential thereof all meet the regulations for minimum standard of MSCidentification from international cell therapy association.
Owner:山西省干细胞基因工程有限公司
Method for establishing goose embryo epithelial cell line and established goose embryo epithelial cell line
ActiveCN104152403AGood growth divisionHigh purityMicroorganism based processesViruses/bacteriophagesBiotechnologyDuck hepatitis A virus
The invention discloses a method for establishing goose embryo epithelial cell line and the established goose embryo epithelial cell line. The invention relates to the method for establishing the goose embryo epithelial cell line, which is characterized in that a primary goose tissue adherent method, a differential velocity enzymatic digestion and a monoclonal screening method are combined, so that primary culture condition can be optimized. The method has the advantages of simple operation process, and convenient popularization and application. The invention also relates to the goose embryo epithelial cell line established by the method, a preservation number of the goose embryo epithelial cell line is CCTCC NO: C2014137, The establishment of the goose embryo epithelial cell line solves the problem of no well-established goose source cell line in prior art. The invention also relates to a kit used for culturing and / or proliferating goose parvovirus, muscovy duck parvovirus and type I duck hepatitis virus and novel duck hepatitis virus, and is characterized in that a host cell to be infected is / or comprises the goose embryo epithelial cell line. The method for establishing the goose embryo epithelial cell line verifies the sensitive characteristic of the goose parvovirus, muscovy duck parvovirus and type I duck hepatitis virus and novel duck hepatitis virus.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com