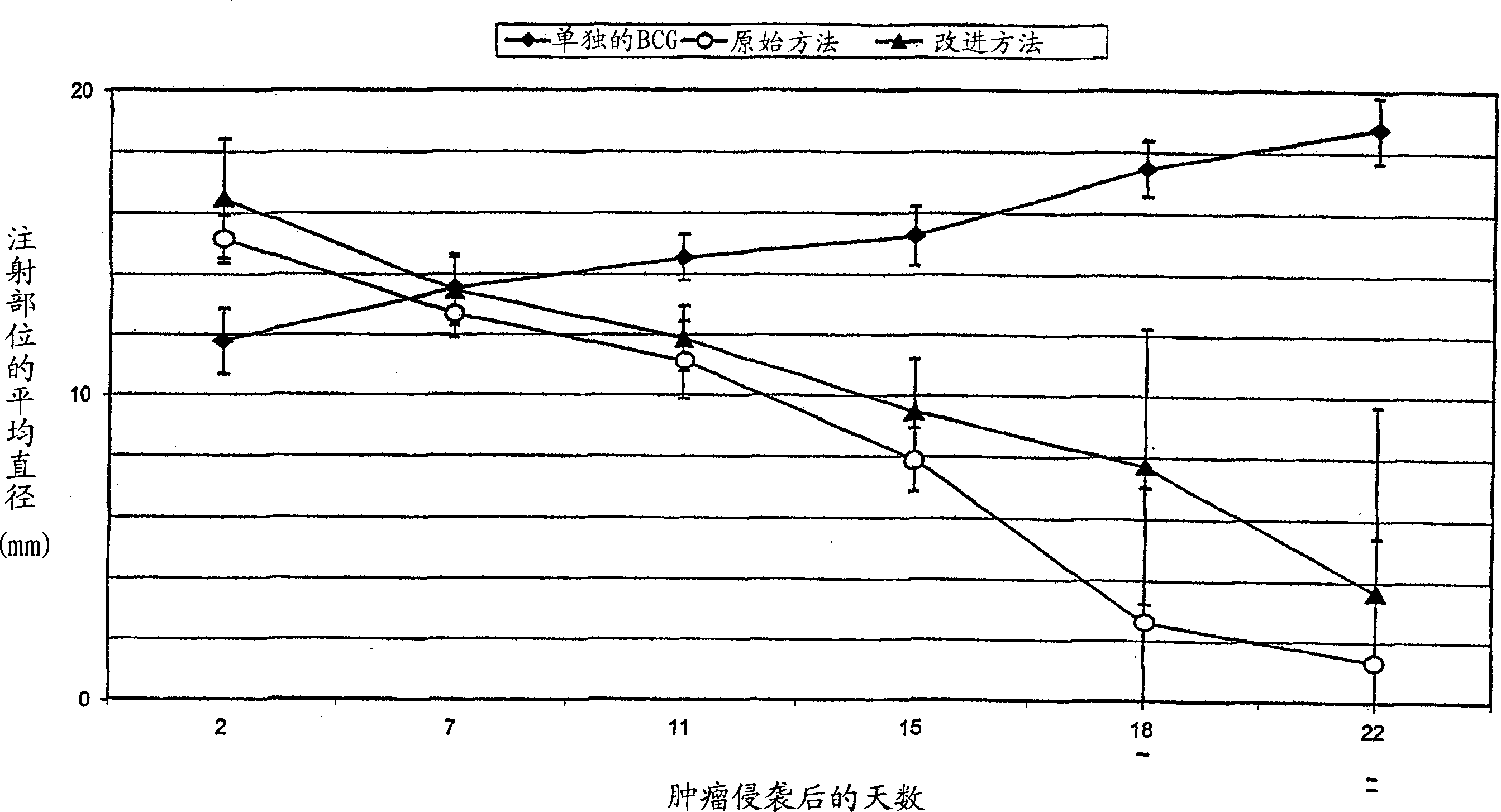
Sterile immunogenic non-tumorigenic tumor cell compositions and methods
A tumor cell, immunogenic technology, applied in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., can solve problems such as changing immunogenicity, cell inactivation, and changing proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Colon and orthotopic tumor washing prior to dissection
[0041] Physiological saline (different from HBSS) for intravenous use is available in 500 mL soft bags. By aseptically fitting a sterile port (eg, a Combi-port) in the bag, the bag can essentially be converted into a spray bottle. By squeezing the bag, a stream of saline is forced directly onto the tumor and surrounding colonic mucosa.
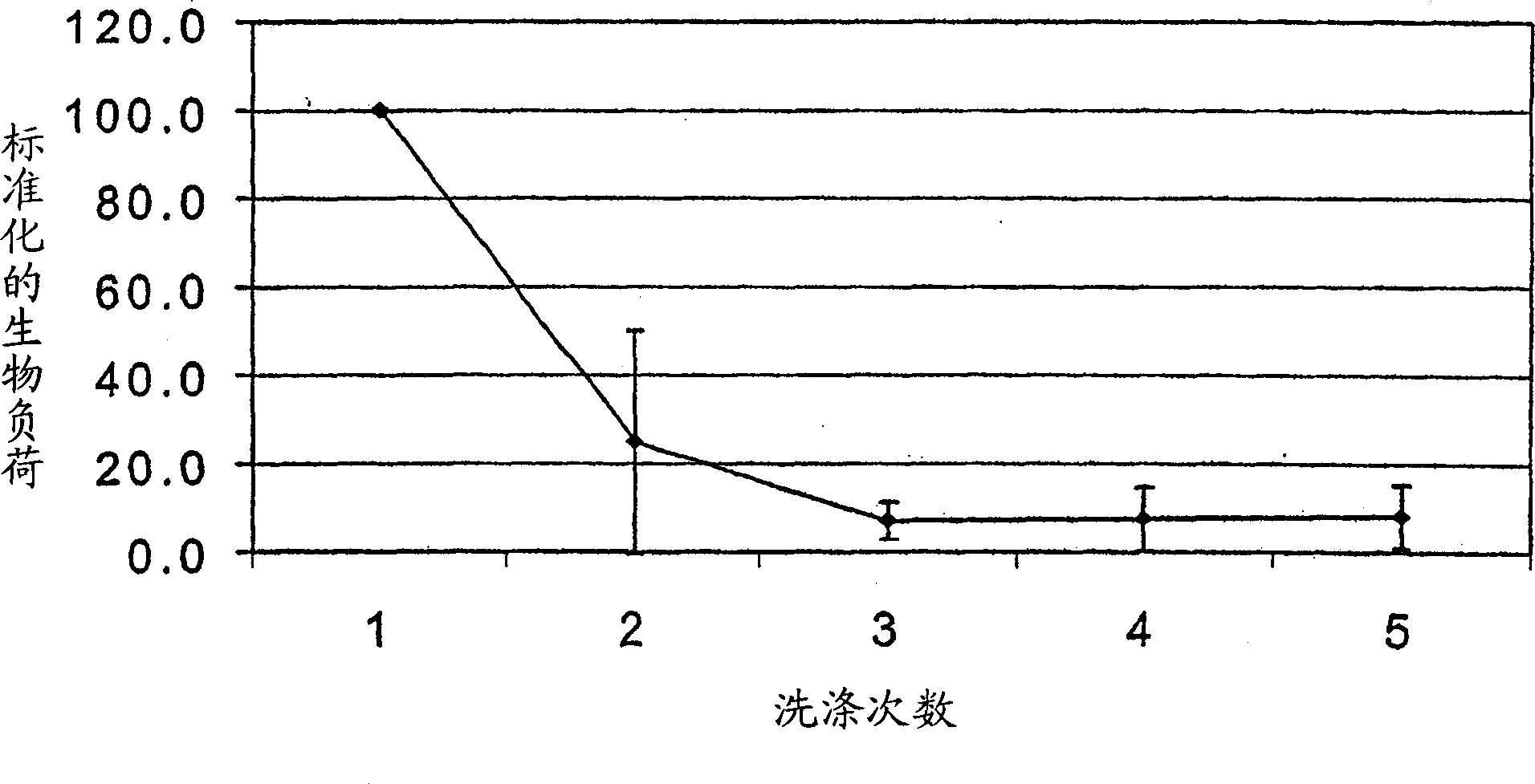
[0042] For the first wash, the colon can be held vertically and washed with 500 mL of normal saline from the top down. In preliminary experiments, the second 500 mL wash consisted of saline or 1% Triton X-100 (dissolved in saline), and was directed at the tumor and its close adjacent areas. This washing is also done when the colon is held vertically. The colon is then held horizontally and the area of the colon containing the tumor is elevated slightly. Tumors were then subjected to further washes (2-3 times), each containing 500 mL of saline. The fluid after e...
Embodiment 2
[0049] Chemical Disinfection of Colon Tumors Before Enzymatic Dissociation
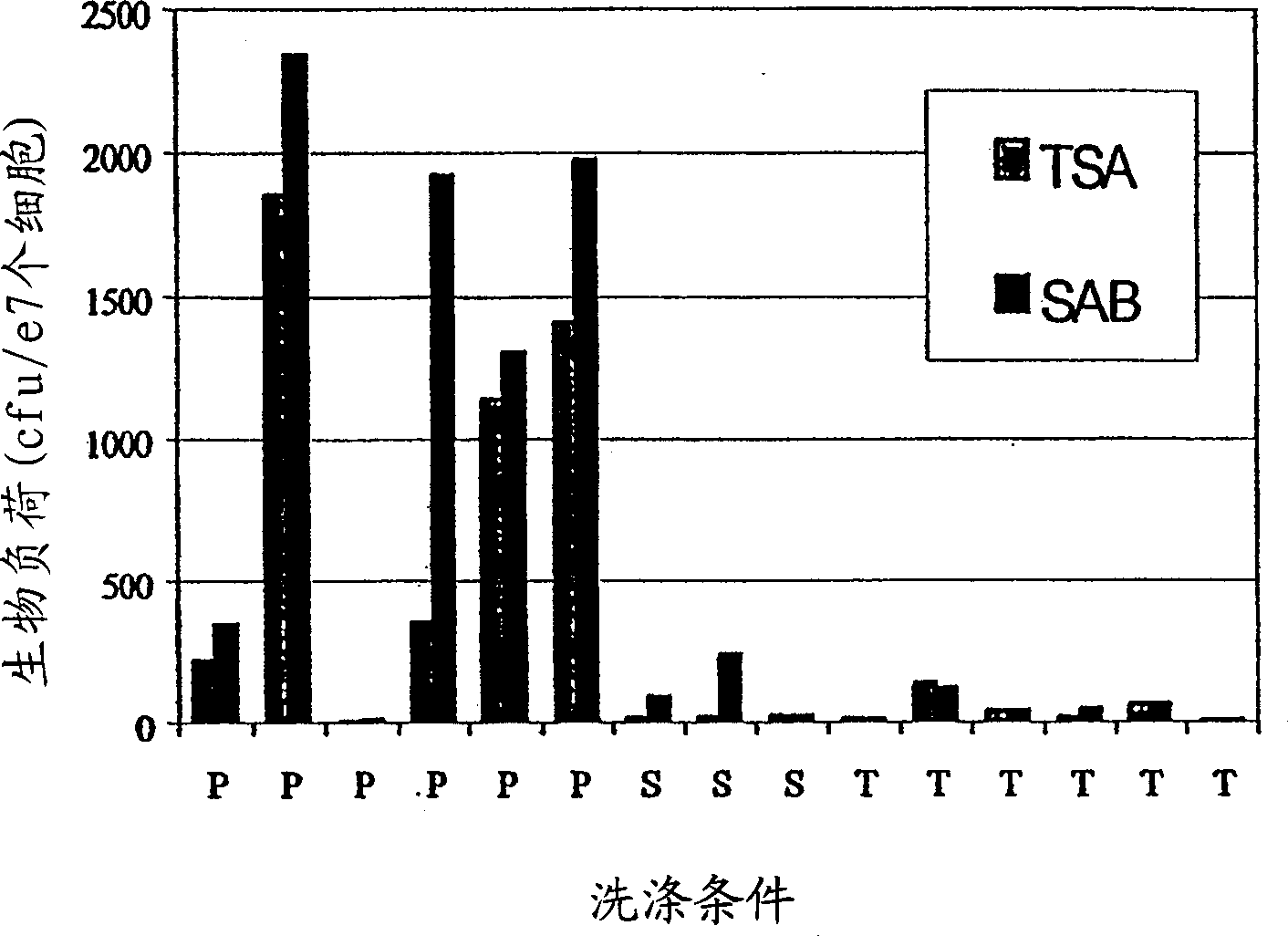
[0050] For pathology stage purposes, pathologists cut tumors to determine the deepest margin of invasion. Therefore, the resected tumor contained several fragments of tumor tissue. The tissue was dissected to remove extraneous non-tumor and necrotic tissue. Trimmed tumor fragments were further subdivided for homogeneity and divided into 2-4 groups for processing according to the experimental design. The tissue was distributed as evenly as possible among the samples so that tumor fragments in the experimental groups looked the same as possible. Trimmed pieces were added to test tubes containing 40 mL of disinfectant and then processed by shaking at 200 rpm for 2 min on a rotary platform shaker. Trimmed tumor fragments were then washed 3 times with HBSS before dissociation. The amount of dissociated tumor was determined by weighing the tumor fragments before dissociation and after comple...
Embodiment 3
[0066] Role of the presence of antibiotics during colon tumor dissociation
[0067] Gut microbes trapped within invaginations in colon tumors are released during enzymatic dissociation. In addition, the enzymatic dissociation process occurs under conditions favorable to the growth of intestinal microorganisms (37 °C). For these reasons, the effect of adding antibiotics to resolvase solutions and other treatment solutions has been investigated.
[0068] Antibiotics are usually used for a longer period of time. The first set of experiments was designed to assess the efficacy of various antibiotics at shorter exposure times. The next set of experiments examined the effectiveness of the antibiotics when included in the resolvase solution.
[0069] Appropriate test microorganisms are incubated with antibiotics at 4°C or 37°C for various periods of time. "Untreated" controls were incubated with HBSS. After treatment, samples were assayed for bioburden by membrane filtrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com