Preparation method of 5-flucytosine
A technology of flucytosine and cytosine, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of highly toxic fluorine gas production operations and production equipment, environmental and operator injuries, unfavorable industrial production, etc., to achieve low cost and avoid Corrosiveness, the effect of product quality stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
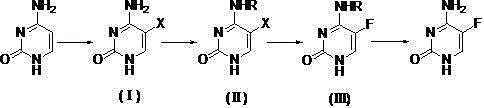
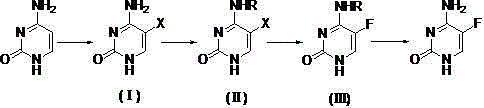
[0023] Embodiment 1: Preparation of 5-bromocytosine
[0024] Suspend cytosine (55.6g, 0.5mol) in 120mL of acetic acid, slowly add a mixed solution of liquid bromine (87.9g, 0.55mol) and 80mL of acetic acid dropwise at room temperature, track by TLC until the reaction of cytosine is complete, and cool in an ice-water bath to 10 ℃, filtered, and the filter cake was washed successively with 20 mL of acetic acid and 20 mL of water, and dried to obtain 5-bromocytosine (89.7 g, 94.3%).
[0025] In this embodiment, the halogenating reagent uses chlorine gas, iodine, N - Chlorosuccinimide, N -Bromosuccinimide, N -Iodosuccinimide, tetrabutylammonium tribromide, tetrabromocyclic ketone, copper bromide or a mixture of several of them instead of liquid bromine; organic solvents use carbon tetrachloride, chloroform, dichloromethane , 1,2-dichloroethane, toluene, xylene, DMF, DMSO, acetonitrile, ethyl acetate, ethanol or a mixture of several of them instead of acetic acid can achieve sim...
Embodiment 2
[0026] Example 2: N - Preparation of acetyl-5-bromocytosine
[0027] Add 5-bromocytosine (95g, 0.5 mol) and acetic anhydride (306.3g, 3mol) into the reaction flask, raise the temperature to 70°C for 4 hours, distill off the solvent under reduced pressure, add 200mL of methanol, raise the temperature to reflux for 30min, and cool to room temperature , filtered, the filter cake was washed with 30mL of methanol, and dried to obtain N - Acetyl-5-bromocytosine (114.3 g, 98.5%).
Embodiment 3
[0028] Example 3: Preparation of 5-fluorocytosine
[0029] Will N -Acetyl-5-bromocytosine (69.6g, 0.3 mol) was put into a dry reaction flask, anhydrous potassium fluoride (23.2g, 0.4mol), acetamide (29.5g, 0.5mol), DMF150mL, heat up to 130°C to react for 5h, evaporate the solvent under reduced pressure, add 150 mL of ammonia methanol after cooling to 40°C and keep the reaction for 12h, trace by TLC until the reaction of intermediate (Ⅲ) is complete, evaporate methanol under reduced pressure, add 400 mL of water Heated until dissolved, cooled to 10°C, filtered, and dried to obtain 5-fluorocytosine (36 g, 93%) as a white crystalline solid, with an HPLC content of 99.5%.
[0030] In this embodiment, sodium fluoride, cesium fluoride, lithium fluoride, antimony trifluoride, antimony pentafluoride, mercury fluoride, silver fluoride, copper fluoride, cobalt fluoride, cerium fluoride , pyridinium hydrogen fluoride, tetrabutylammonium fluoride, tetramethylammonium fluoride or carrier...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com