Formulations of 5-fluorocytosine and uses thereof
A preparation, 5-FC technology, applied in the field of 5-flucytosine preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
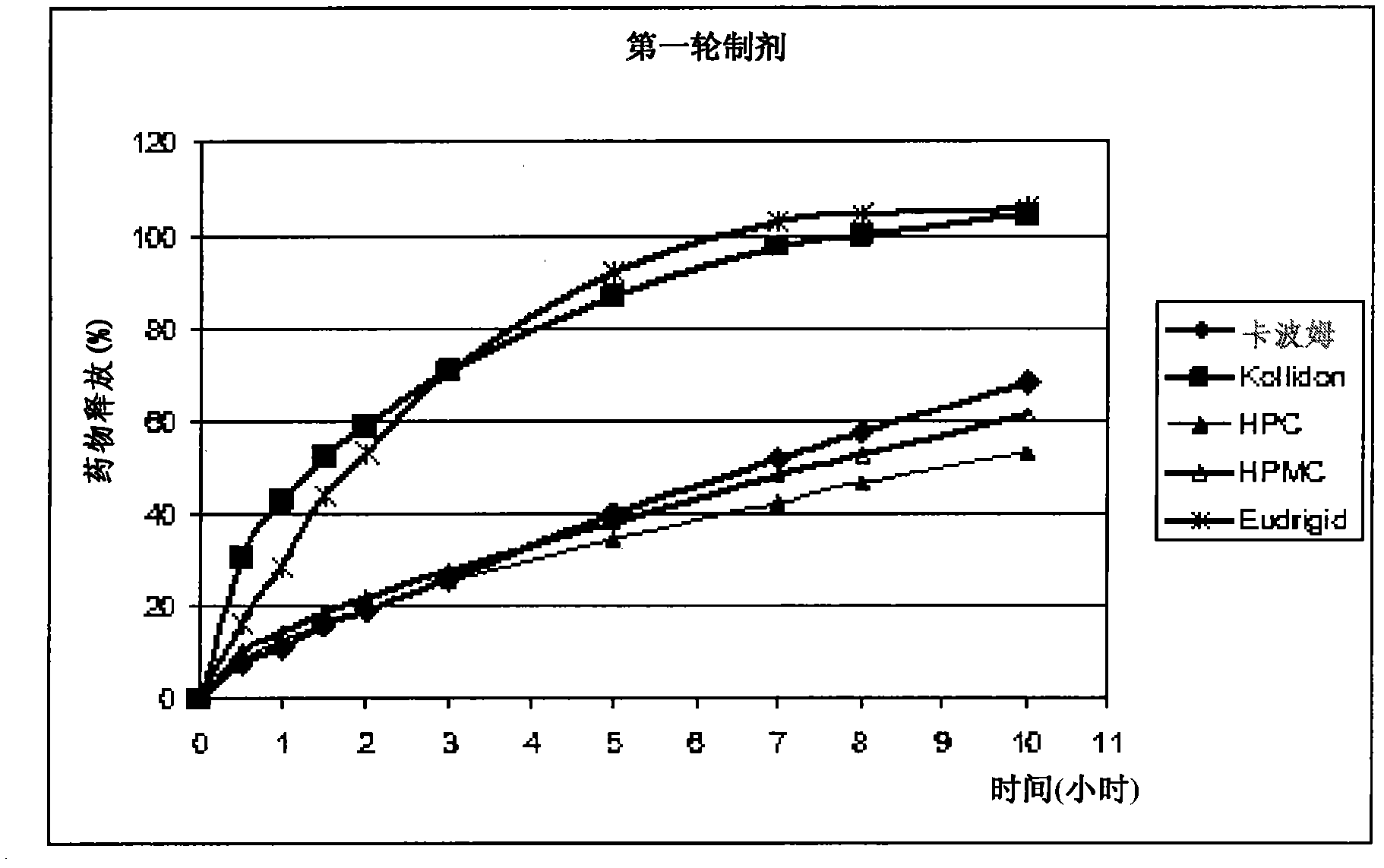
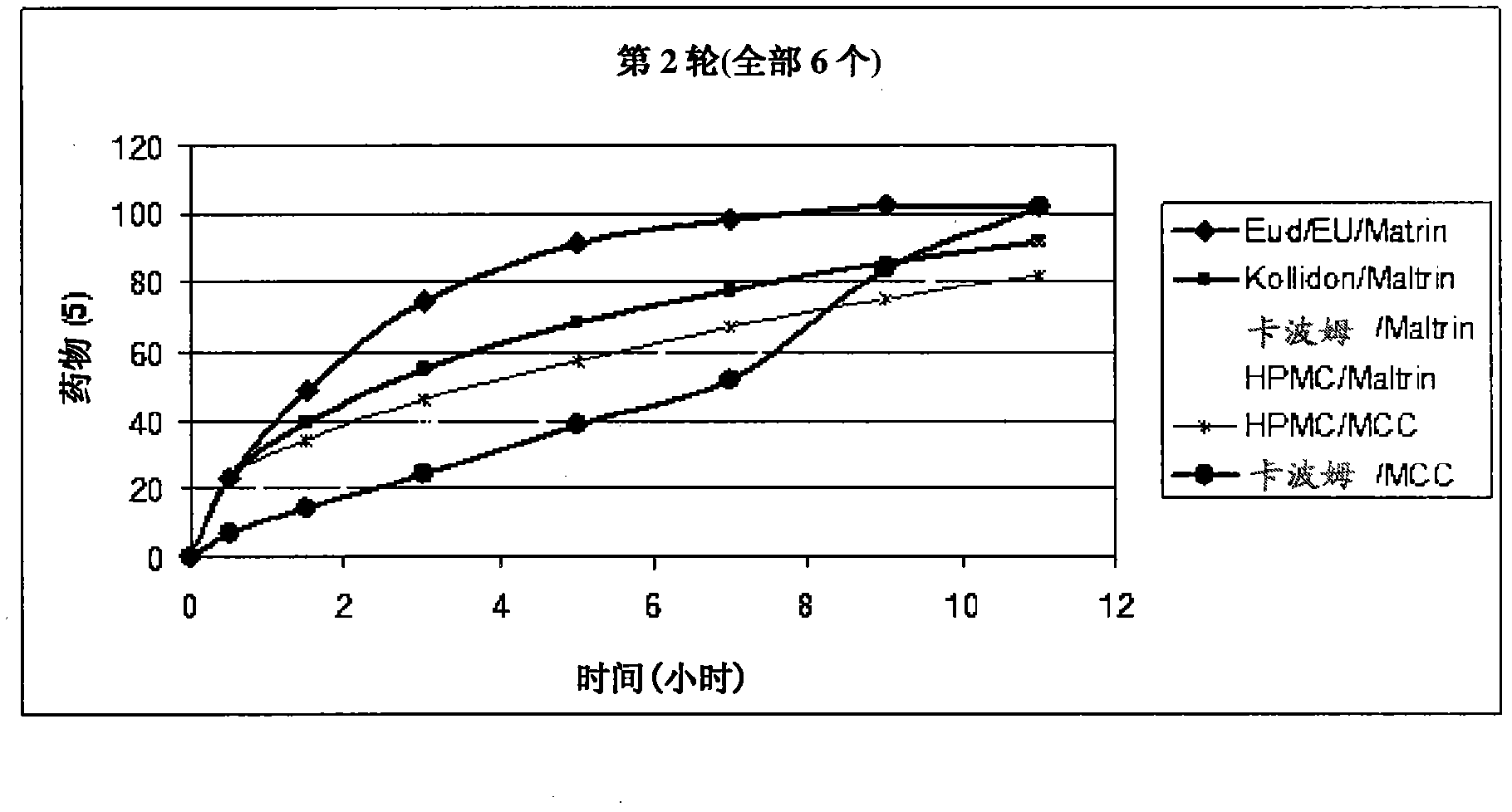
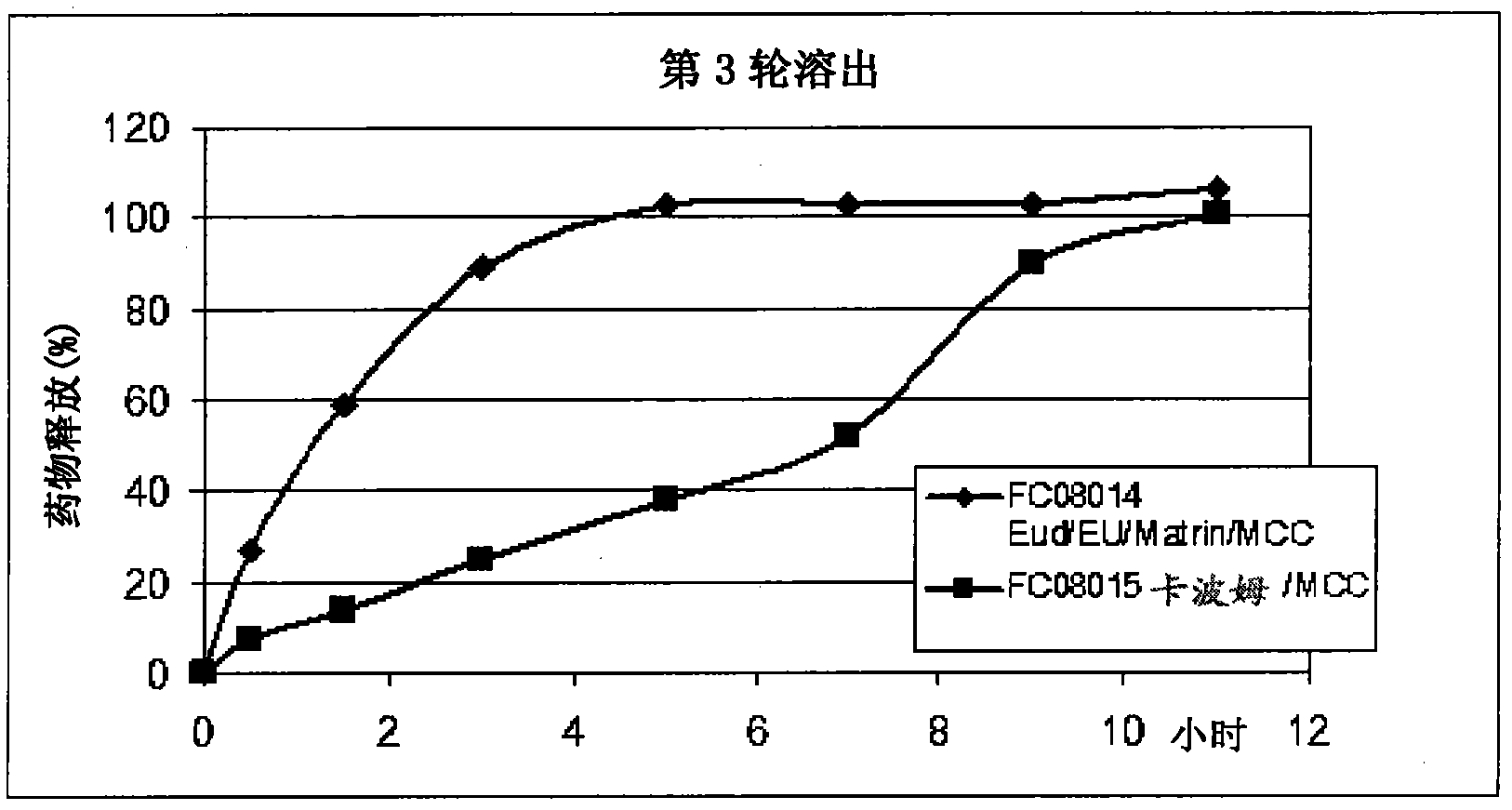
Image
Examples
Embodiment 1
[0100] Materials. Materials used in the preparation of the dosage forms described herein were obtained as follows. Flucytosine (5-FC), USP was purchased from Scinopharm (Taiwan); Carbomer 71-G was purchased from Noveon (Cleveland, Ohio, USA); HPMC, NF (Benecel MP874, hydroxypropyl methylcellulose) and HPC, NF (Klucel -HXF, hydroxypropyl cellulose) was purchased from Aqualon (Wilmington, Delaware, USA); Kollidon - SR (PVA-PVP copolymer, matrix retarding polymer based on polyvinyl acetate and povidone) was purchased from BASF (Ludwigshafen am Rhein, Rhineland-Palatinate, Germany); directly compressible dicalcium phosphate, NF (Emcompress ) was purchased from JRS Pharma (Patterson, New York, U.S.); maltodextrin was purchased from Grains Processing Corp. (GPC, Muscatine, Iowa); magnesium stearate NF was purchased from Spectrum Chemicals (Gardena, California, U.S.) and could be directly Compressed anhydrous lactose (DCL-21) was purchased from DMV (Veghel, Netherlands). Sodi...
Embodiment 2
[0106] Repeat the above method using the following ingredients:
[0107] Element
Embodiment 3
[0109] Repeat the above method using the following ingredients:
[0110] Element
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com