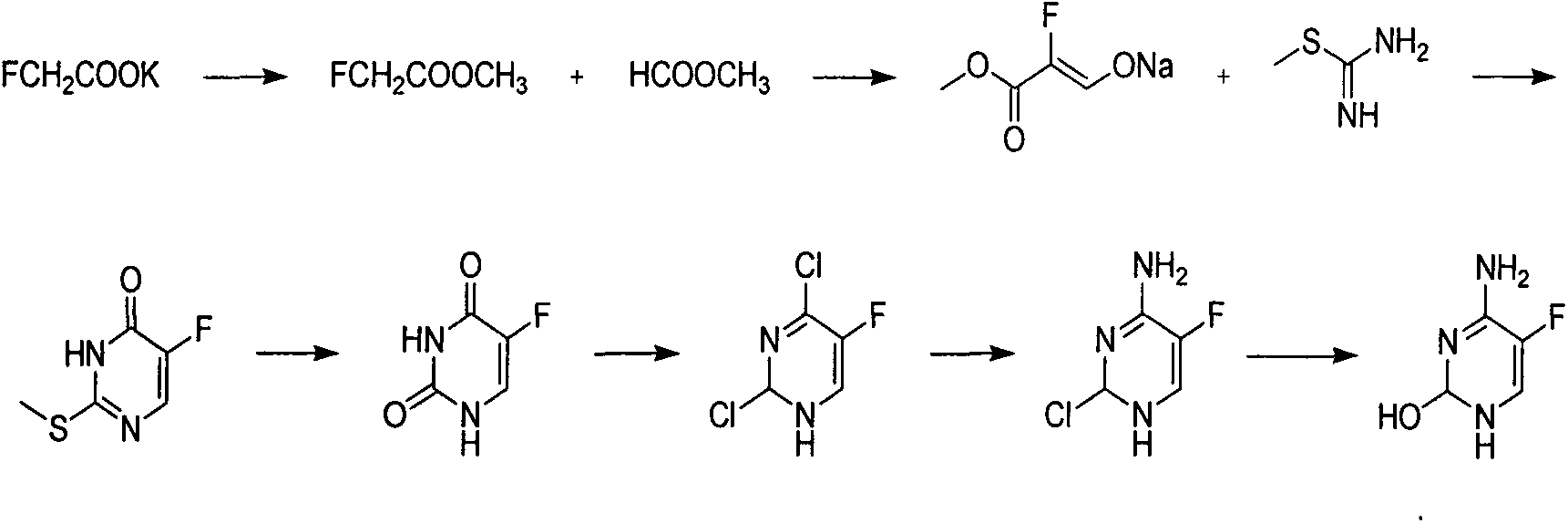
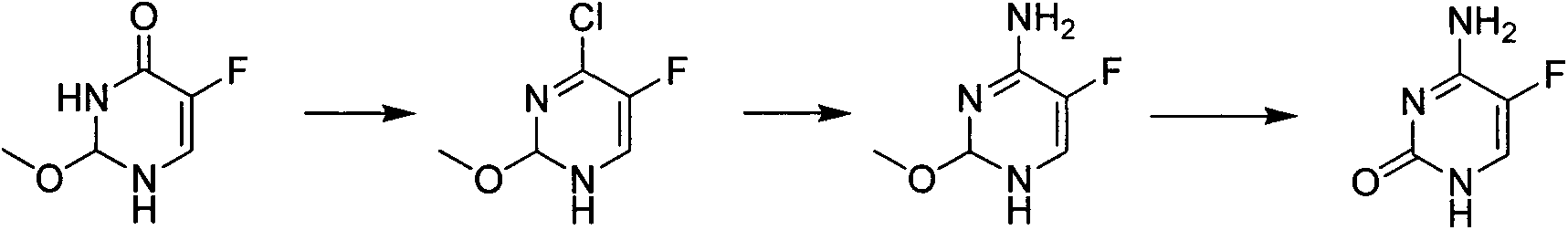
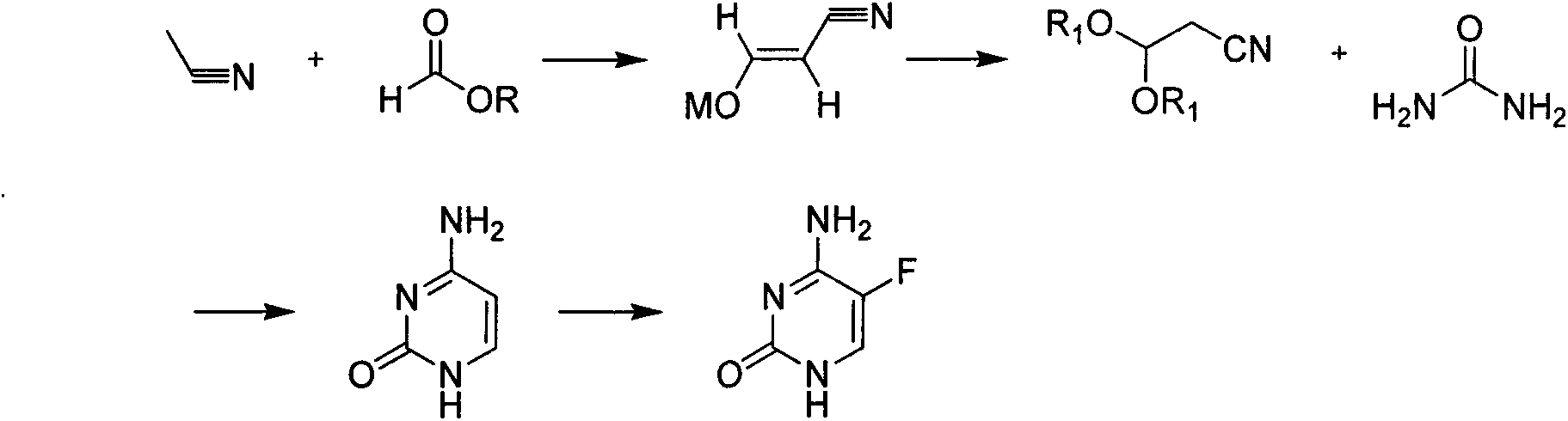
Novel technology for synthesis of 5-flucytosine
A technology of flucytosine and new process, applied in the field of synthesizing 5-flucytosine, can solve the problems of difficult procurement, unstable product quality, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 1500kg of methyl tetrahydrofuran, 180kg of sodium ethoxide, 100kg of acetonitrile and 190kg of methyl formate in sequence in a 3000L reactor, heat up and reflux for 56 hours, cool down to 0-5°C, and set aside. In another 5000L reactor, add 1300kg methyl tetrahydrofuran, 800kg hydrochloric acid ethanol solution, 100kg anhydrous sodium sulfate in sequence, cool down to 0-5°C, add the solution obtained in the previous step into the reactor, dropwise add the process control kettle The internal temperature should not exceed 10°C. After the dropwise addition, raise the temperature to 30-35°C, keep the temperature for 6 hours, cool down to 0-5°C, add baking soda in batches, adjust the pH of the system to be neutral, centrifuge, and rectify the filtrate 230 kg of 3,3-diethoxypropionitrile were obtained. Add 800kg of toluene, 150kg of sodium methoxide and 150kg of urea in the 2000L reactor in sequence, raise the temperature to 60-65°C, keep warm and slowly add 230kg of 3,3-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com