Pneumocandin B0 purification method
A purification method, the technology of neomocontin, which is applied in the chemical field, can solve the problems of product quality, yield reduction, troublesome production operation, and long time required, so as to avoid product quality and yield reduction, small product damage, and dead adsorption Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
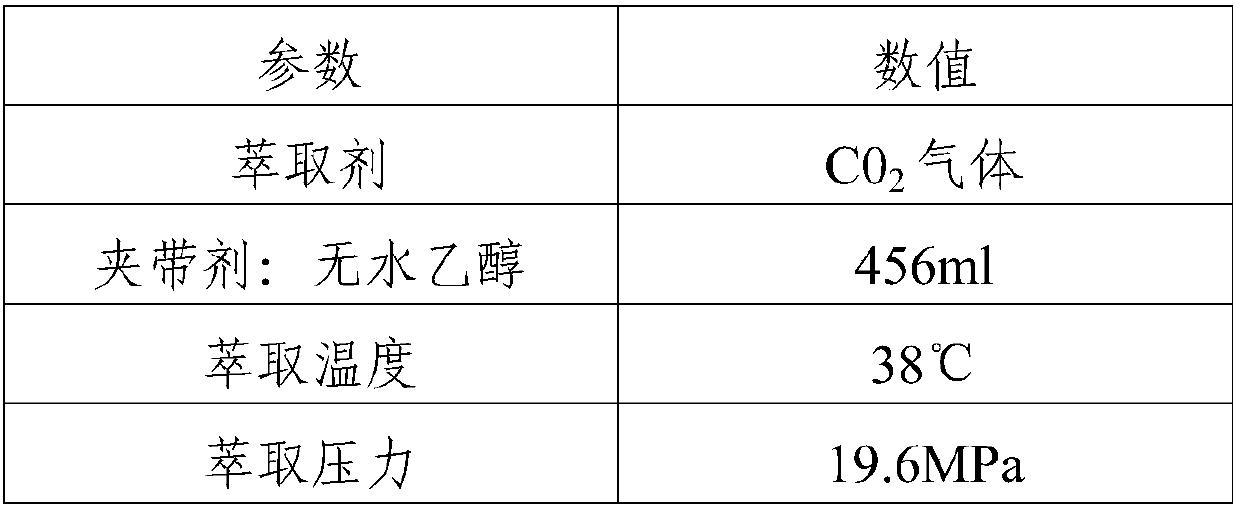
[0050] A method for purifying pneumocidine B0, comprising the steps of:
[0051] (1) Fermentation tank, 152L fermented liquid is carried out solid-liquid separation to obtain the bacterium slag containing PBO, be 70% methanol aqueous solution 450L extraction bacterium slag with methanol content, solid-liquid separation obtains extract; Repeat extraction 2 times again, merge All the extracts are then clarified by a high-speed tube centrifuge, and the clarified liquid is then filtered through a liquid filter with a filter element pore size of 0.54um;
[0052] (2) The liquid filtered by step (1) is filtered by an ultrafiltration device with an ultrafiltration membrane molecular weight of 5000 Daltons, then concentrated by a nanofiltration device with a nanofiltration membrane molecular weight of 980 Daltons, using methanol as a displacing agent, and continuing Adopt the nanofiltration equipment of 980 Daltons to process the nanofiltration liquid, make the concentration of methano...
Embodiment 2
[0063] A method for purifying pneumocidine B0, comprising the steps of:
[0064] (1) Fermentation tank, 223L fermented liquid is carried out solid-liquid separation to obtain the bacterium residue that contains PBO, be 80% methanol aqueous solution 500L extraction bacterium residue with methanol content, solid-liquid separation obtains extract; Repeat extraction 2 times again, merge All the extracts are then clarified by a high-speed tube centrifuge, and the clarified liquid is then filtered through a liquid filter with a filter element pore size of 0.54um;
[0065] (2) The liquid filtered by step (1) is filtered by an ultrafiltration device with an ultrafiltration membrane molecular weight of 5000 Daltons, then concentrated by a nanofiltration device with a nanofiltration membrane molecular weight of 900 Daltons, using methanol as a displacing agent, and continuing Adopt the nanofiltration equipment of 900 Daltons to process the nanofiltration liquid, make the concentration o...
Embodiment 3
[0076] A method for purifying pneumocidine B0, comprising the steps of:
[0077] (1) Fermentation tank, 132L fermented liquid is carried out solid-liquid separation to obtain the bacterium residue that contains PBO, be the ethanol aqueous solution 400L extraction bacterium residue of 50% with methanol content, solid-liquid separation obtains extract; Repeat extraction 2 times again, merge All the extracts are then clarified by a high-speed tube centrifuge, and the clarified liquid is then filtered through a liquid filter with a filter element pore size of 0.54um;
[0078] (2) The liquid filtered by step (1) is filtered by an ultrafiltration device with an ultrafiltration membrane molecular weight of 5000 Daltons, then concentrated by a nanofiltration device with a nanofiltration membrane molecular weight of 980 Daltons, using methanol as a displacing agent, and continuing Adopt the nanofiltration equipment of 980 Daltons to process the nanofiltration liquid, make the concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com