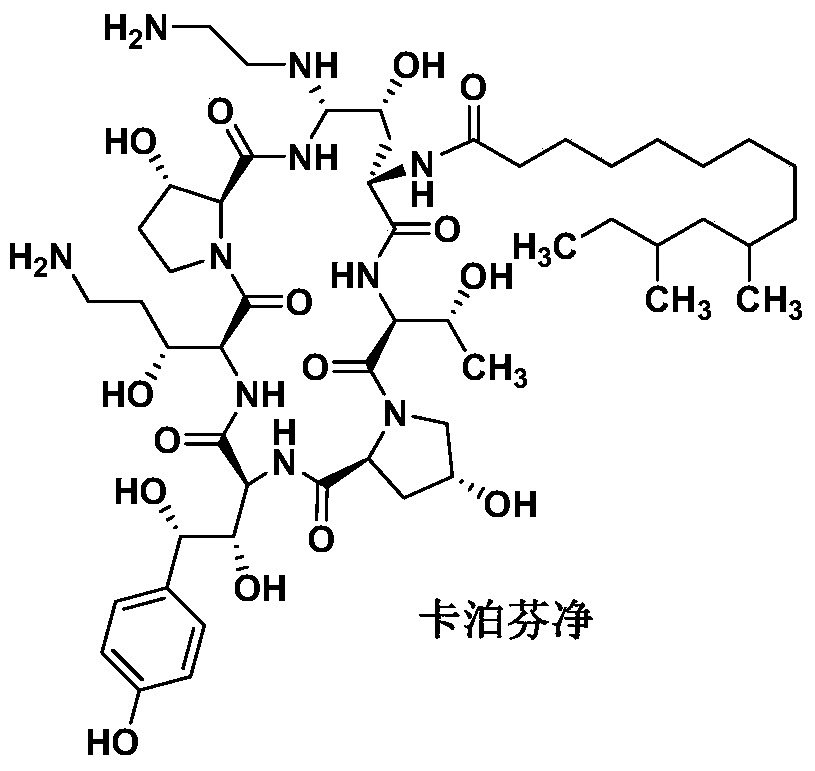
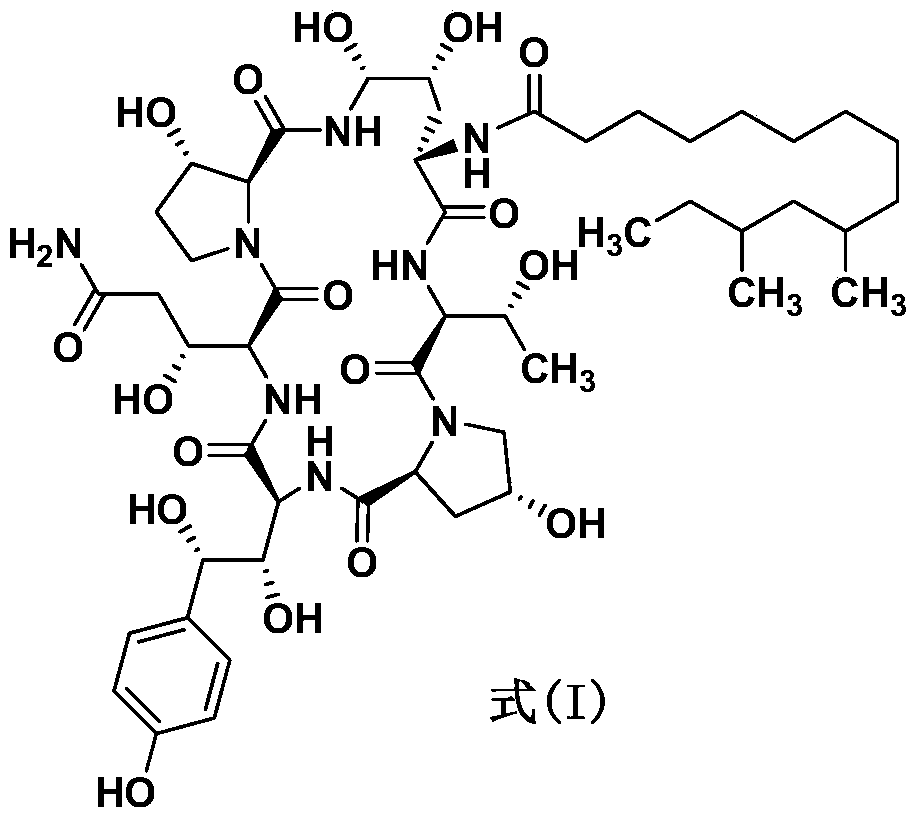
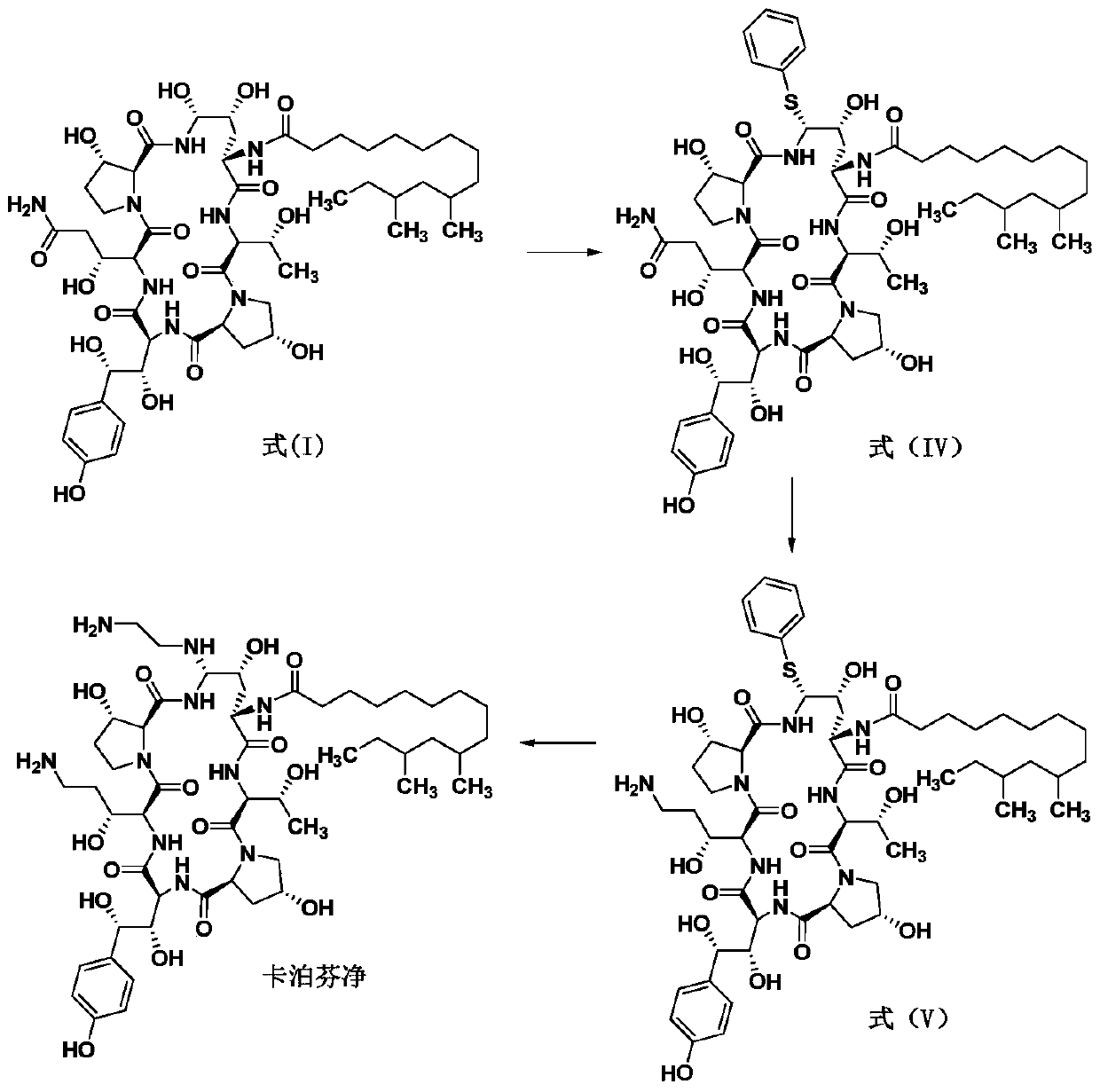
The preparation method of caspofungin
A technology of caspofungin and compounds, applied in the field of preparation of caspofungin, can solve the problems of complex operation, long time required, and easy degradation, etc., and achieve the effects of reducing reaction time, simplifying operation, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 caspofungin
[0026]
[0027] Under stirring, add 47.5kg of acetonitrile into a 100L reaction tank, pass through nitrogen protection, add 2.0kg of the compound of formula (I) and 458g of phenylboronic acid, stir for 10 minutes, add 700g of p-cresyl thiophenol, cool down, and the temperature of the feed liquid is controlled at - After reacting at 20~-15°C for 2 hours, 845 g of trifluoromethanesulfonic acid was slowly added dropwise. During the dropping process, the temperature of the feed liquid was controlled at -20 to -15°C. After the dropwise addition, the temperature of the feed liquid was controlled at -20 to -15°C and the reaction was stirred for 4 hours. HPLC followed and monitored until the basic reaction of the raw materials was complete. After the reaction, add sodium acetate solution (462g of anhydrous sodium acetate is prepared by adding 6.8kg of water), heat up, control the temperature of the feed solution at 20-30°C and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com