Preparation method of high-purity and high-yield caspofungin impurity CO
A caspofungin, high-yield technology, applied in the chemical field, can solve the problem of how to obtain high-purity caspofungin impurities in large quantities, and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
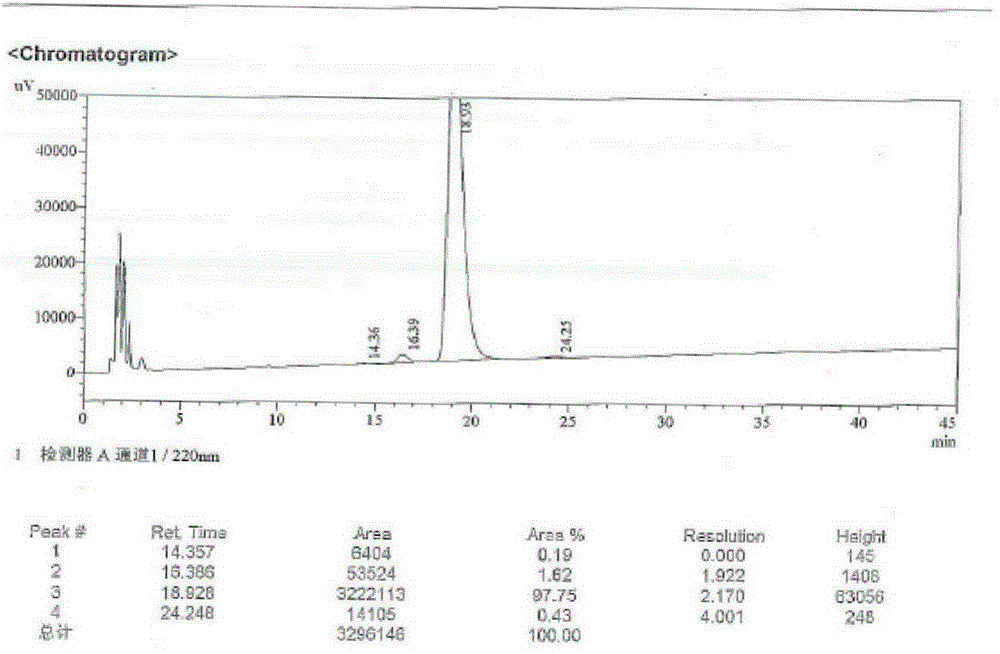
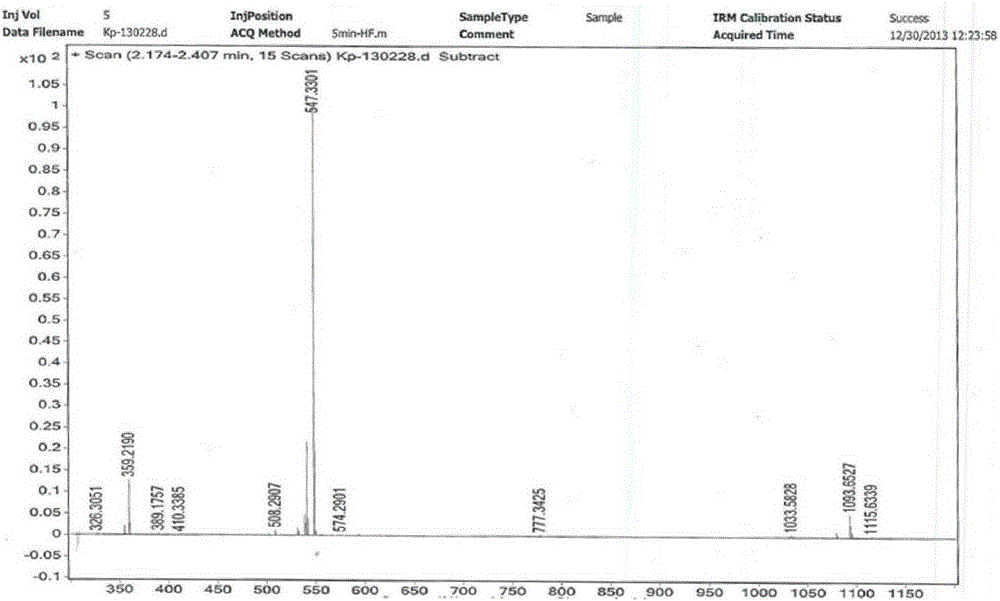
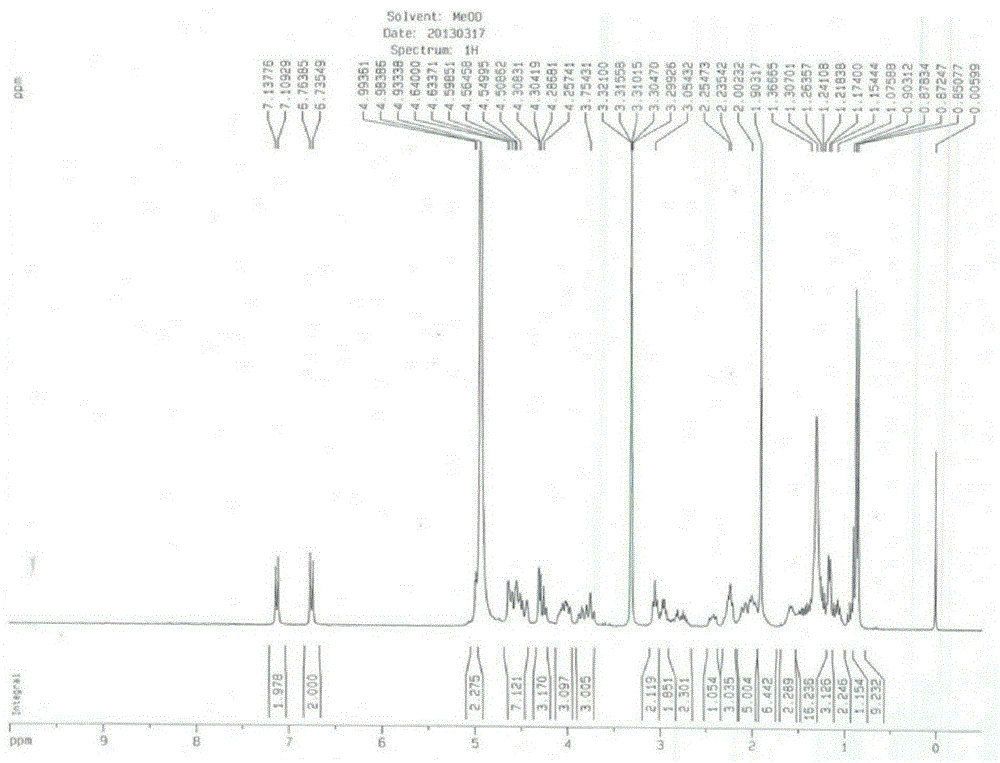
[0022] Step 1: Add 100ml of acetonitrile into a 250ml three-necked bottle, and mix 10g of neomocontin B with stirring 0 and C 0 The solid (HPLC purity B 0 +C 0 96.1%, B 0 / C 0 =80 / 20), 2.3g of phenylboronic acid, 3.1g of thiophenol, placed in an external bath at -25 to -20°C, when the internal temperature dropped to -20°C, slowly add 4.3g of trifluoromethanesulfonic acid dropwise, and dropwise After completion, the reaction was continued for 3-4 hours, and the reaction was detected by TLC (EA / MeOH / 10% glacial acetic acid=82 / 8 / 8). After the basic reaction of the raw materials was complete, the reaction was stopped. Add sodium acetate aqueous solution (2.3g / 23ml) dropwise to the three-necked flask, and a large amount of white solids gradually precipitated. After the dropwise addition, stirred for 1 hour, filtered, and the solid was air-dried at 40°C to obtain 9.8g of intermediate I. HPLC Purity 91.6% (B 0 +C 0 91.6%, B 0 / C 0 =82 / 18), yield 90.2%.
[0023] Step 2: Add ...
Embodiment 2
[0026] Step 1: Add 100ml of acetonitrile into a 250ml three-necked bottle, and mix 10g of neomocontin B with stirring 0 and C 0 The solid (HPLC purity B 0 +C 0 95.1%, B 0 / C 0 =71 / 29), 2.3g of phenylboronic acid, 3.1g of thiophenol, placed in an external bath at -25 to -20°C, when the internal temperature dropped to -20°C, slowly add 4.3g of trifluoromethanesulfonic acid dropwise, and dropwise After completion, the reaction was continued for 3-4 hours, and the reaction was detected by TLC (EA / MeOH / 10% acetic acid=82 / 8 / 8). After the basic reaction of the raw materials was complete, the reaction was stopped. Add sodium acetate aqueous solution (2.3g / 23ml) dropwise to the three-necked bottle, and a large amount of white solids gradually precipitated. After the dropwise addition, stirred for 1 hour, filtered, and the solid was air-dried at 40°C to obtain 10.3g of intermediate Ⅰ, HPLC Purity 90.3% (B 0 +C 0 90.3%, B 0 / C 0 =72 / 28), yield 94.5%.
[0027] Step 2: Add 100ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com