Preparation method for high-purity echinocandin compound
A technology of echinocandin and compound, which is applied in the field of preparation of high-purity echinocandin compound FR179642, can solve the problem of no separation and purification process, etc., and achieve the effects of shortening process cycle, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
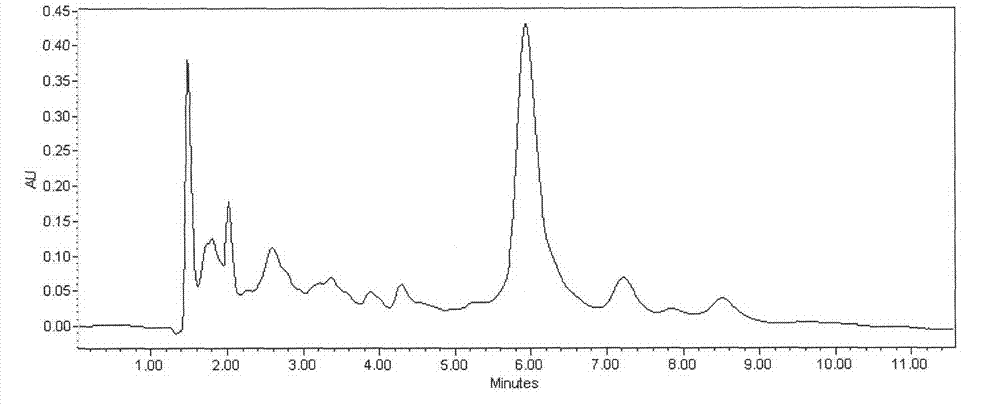
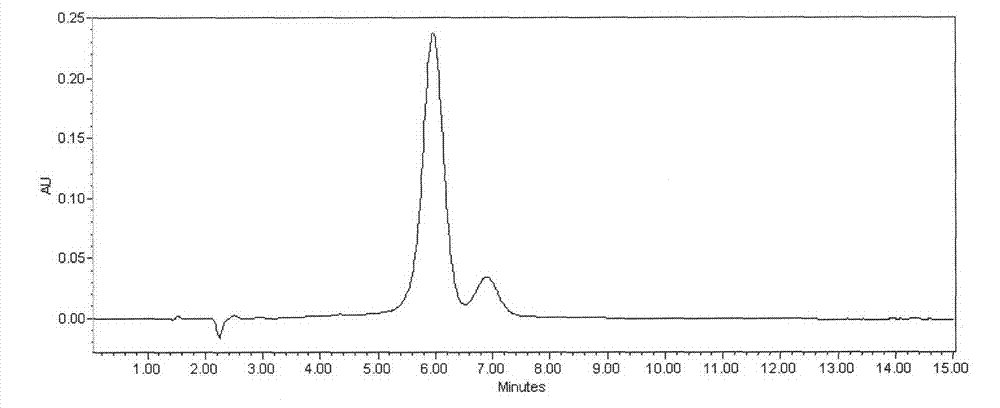
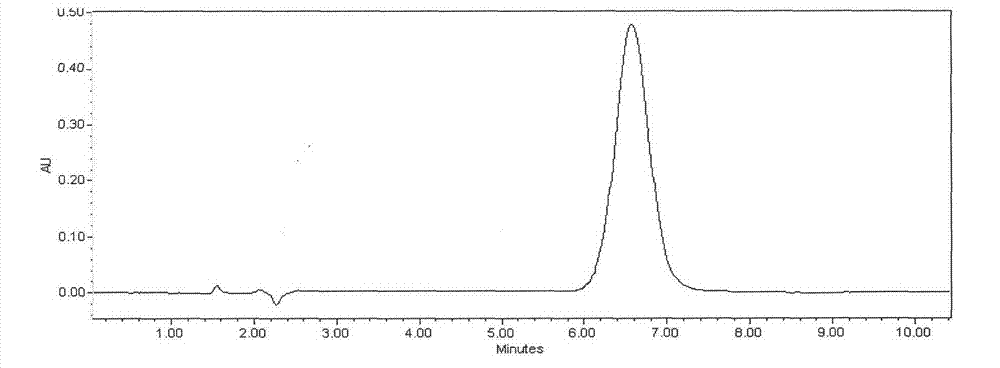
[0035] Take FR179642 fermentation broth 20.0L, fermentation unit 520μg / mL (9.67g). Add 1kg perlite to the fermented liquid, vacuum filter after stirring for 30min, obtain 16.8L clarified filtrate (see attached figure 1 ). The filtrate was introduced into an LX-18 adsorption resin column (Φ8×50cm), with a loading capacity of 1400mL and a sample loading flow rate of 1400mL / h. The saturated resin was purified and washed with deionized water, and then desorbed with 10% acetone at a desorption flow rate of 470 mL / h. The desorption solution was adjusted to PH=4.0 with glacial acetic acid, then concentrated to 193mL, slowly added 1930mL of acetone for powder crystallization, static, and filtered to obtain 12.82g of crude extract of FR179642 (see attached figure 2 ). The crude extract of FR179642 was separated by polymer microsphere chromatography with acetone-water gradient elution. The eluate was concentrated, crystallized, and dried in sections (at a temperature of 40°C and a ...
Embodiment 2
[0037] Take FR179642 fermentation broth 100.0L, fermentation unit 723μg / mL (72.3g). Add 7kg diatomaceous earth in fermented liquid, vacuum suction filtration after stirring 30min, obtain 84.3L clarification filtrate (with attached figure 1 similar peak shape). The filtrate was introduced into an AB-8 adsorption resin column (Φ14×80 cm), with a loading capacity of 8.5 L and a sample loading flow rate of 17 L / h. The saturated resin was purified and washed with deionized water, and then desorbed with 40% methanol at a desorption flow rate of 3.5 L / h. The desorption solution was adjusted to PH=4.5 with oxalic acid, then concentrated to 723mL, slowly added to 5784mL of methanol for powder crystallization, static and filtered to obtain 105.7g of crude extract of FR179642 (with the attached figure 2 similar peak shape). ODS C for crude extract of FR179642 18 Chromatographic separation, methanol-water gradient elution. The eluate was concentrated, crystallized, and dried in sect...
Embodiment 3
[0039] Take FR179642 fermentation broth 500.0L, fermentation unit 643μg / mL (321.5g). Add 50kg perlite in fermented liquid, vacuum suction filtration after stirring 30min, obtain 427L clarification filtrate (with attached figure 1 similar peak shape). The filtrate was introduced into an LX-18 adsorption resin column (Φ24×140cm), with a loading capacity of 50L and a sample loading flow rate of 75L / h. The saturated resin was purified and washed with deionized water, and then desorbed with 25% ethanol at a desorption flow rate of 25 L / h. The desorption solution was adjusted to PH=5.0 with hydrochloric acid, then concentrated to 4.0L, slowly added to 24L of absolute ethanol for powder crystallization, static and filtered to obtain 460g of crude extract of FR179642 (with the attached figure 2 similar peak shape). The crude extract of FR179642 was separated by polymer microsphere chromatography and ethanol-water gradient elution. The eluate was concentrated, crystallized, and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com