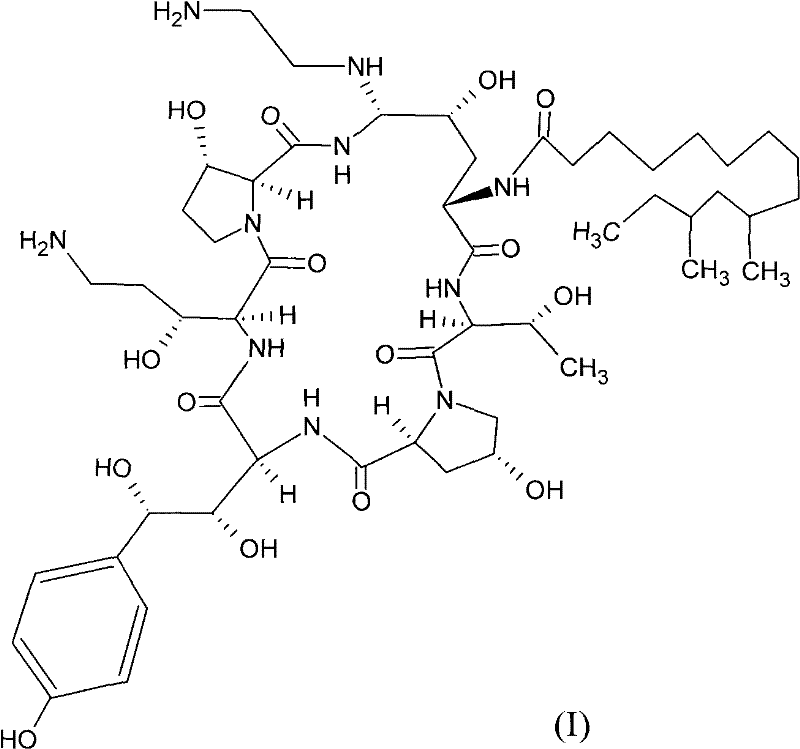
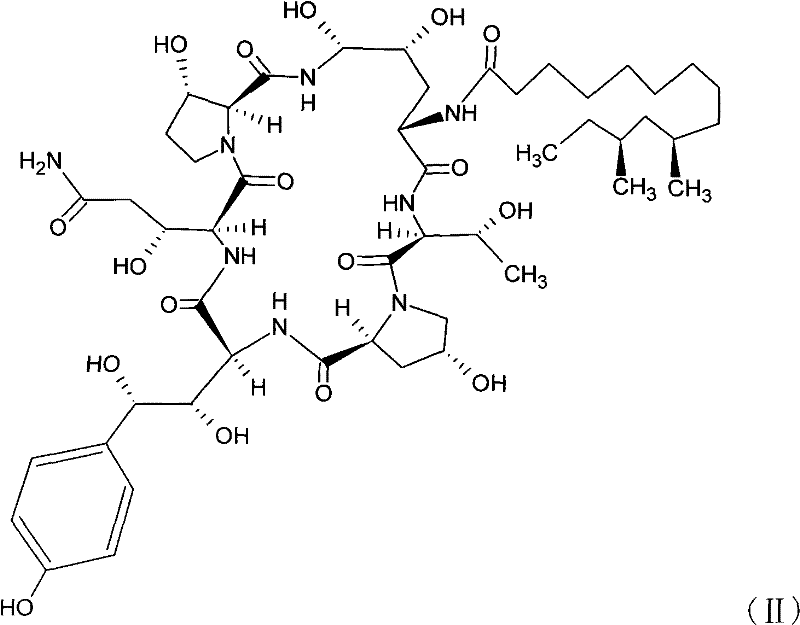
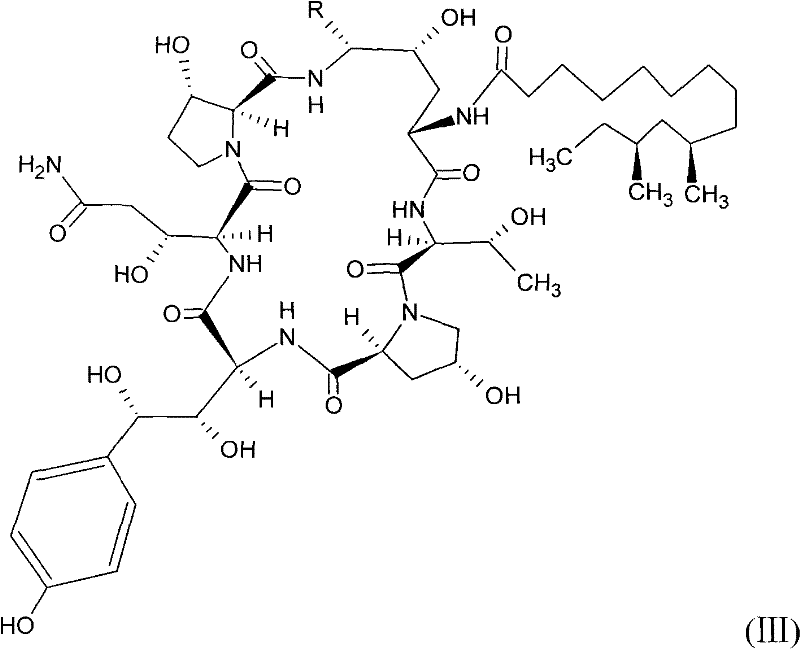
Safer preparation method of echinocandin antifungal medicament
A compound, caspofungin technology, applied in the direction of peptides, etc., can solve the problems of odor and irritation, unsuitable for industrial production, etc., and achieve the effect of safety in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The reaction process is as follows:
[0028]
[0029] Neomercontin B0(1-1)
[0030]
[0031]
[0032] Process flow:
[0033] Compound (1-1) neomocontin B0 (150.20g, 141.0mmol), trifluoromethanesulfonic acid (37.35mL, 423mmol), phenylboronic acid (34.38g, 282mmol), 2-mercaptobenzothiazole (70.74g, 423mmol ) was added to anhydrous acetonitrile (4.5 L). The suspension was cooled to -15°C and kept, detected by HPLC, until the reaction was complete (2.5h). The reaction product was cooled rapidly, and 450mL NaAc·3H 2 O (57.45 g, 423 mmol) in water precipitated the product. The suspension was warmed to room temperature for 2 h, then cooled to below 0 °C. The product was isolated by filtration, and the product was purified by chromatography, eluting with 1:9 (v / v) H2O / CH3CN to obtain the eluate of compound (1-2), which was concentrated and dried to obtain 163.98 g of compound (1-2), which was measured by HPLC. Its purity is 94% (with reference to the standard), a...
Embodiment 2
[0044] The reaction process is as follows:
[0045]
[0046] Neomercontin B0(2-1)
[0047]
[0048]
[0049] Process flow:
[0050] Compound (2-1) Neomocontin B0120g, 112.8mmol), trifluoromethanesulfonic acid (29.88mL, 338.4mmol), phenylboronic acid (27.48g, 225.6mmol), 1-phenyl-5-mercapto-tetrazolium (60.24 g, 338.4 mmol) was added to anhydrous acetonitrile (3.6 L). The suspension was cooled to -15°C and kept, detected by HPLC, until the reaction was complete (2.5h). The reaction product was rapidly cooled and added to 399.6mL NaAc·3H 2 The product was precipitated from aqueous O (45.96 g, 338.4 mmol). The suspension was warmed to room temperature for 2 h, then cooled to below 0 °C. The product was isolated by filtration and the product was purified by chromatography, 1:9 (v / v) H2O / CH 3 CN was eluted to obtain the eluate of compound (2-2), which was concentrated to obtain 135.25 g of compound (2-2), the purity of which was measured by HPLC was 93% (with the sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com