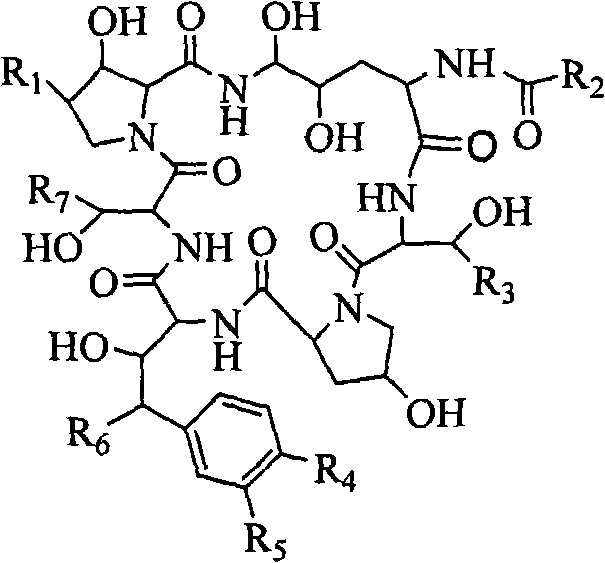
Echinocandin biotransformation method
A biotransformation and echinocandin technology, applied in the direction of biochemical equipment and methods, microorganism-based methods, microorganisms, etc., can solve the problems of affecting the transformation effect, reducing the transformation effect, cell damage, etc., and shorten the transformation cycle and reduce the Production cost, effect of increased substrate concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Cultivation and preparation of whole cell catalyst
[0034] The microorganism is Actinoplanes utahensis AS 4.1543.
[0035] Slant medium: Chase-Peptone Agar
[0036] Seed medium (%): glucose 2, maltodextrin 1, cottonseed cake powder 0.5, yeast powder 1, peptone 0.5, CaCO 3 0.1.
[0037] Fermentation medium (%): sucrose 4, cottonseed cake powder 3, yeast powder 1, K 2 HPO 4 0.1, CaCO 3 0.2, KCl 0.2, MgSO 4 ·7H 2 O 0.1.
[0038] Culture method: culture on slant at 28°C for 7-10d, pick up 0.5×1cm colony with inoculation shovel and inoculate in 25mL / 250mL seed medium, culture at 28°C, 200-250r / min for 48-60h, as fermented seeds . The above-mentioned seeds are inoculated in the fermentation medium according to the inoculum amount (v / v) of 2-10%, and cultured for 72-120 hours at 27-32° C. and 200-250 r / min.
[0039] Preparation of the whole-cell catalyst: centrifuge the fermented broth obtained from fermentation, collect the cells, and then wash severa...
Embodiment 4
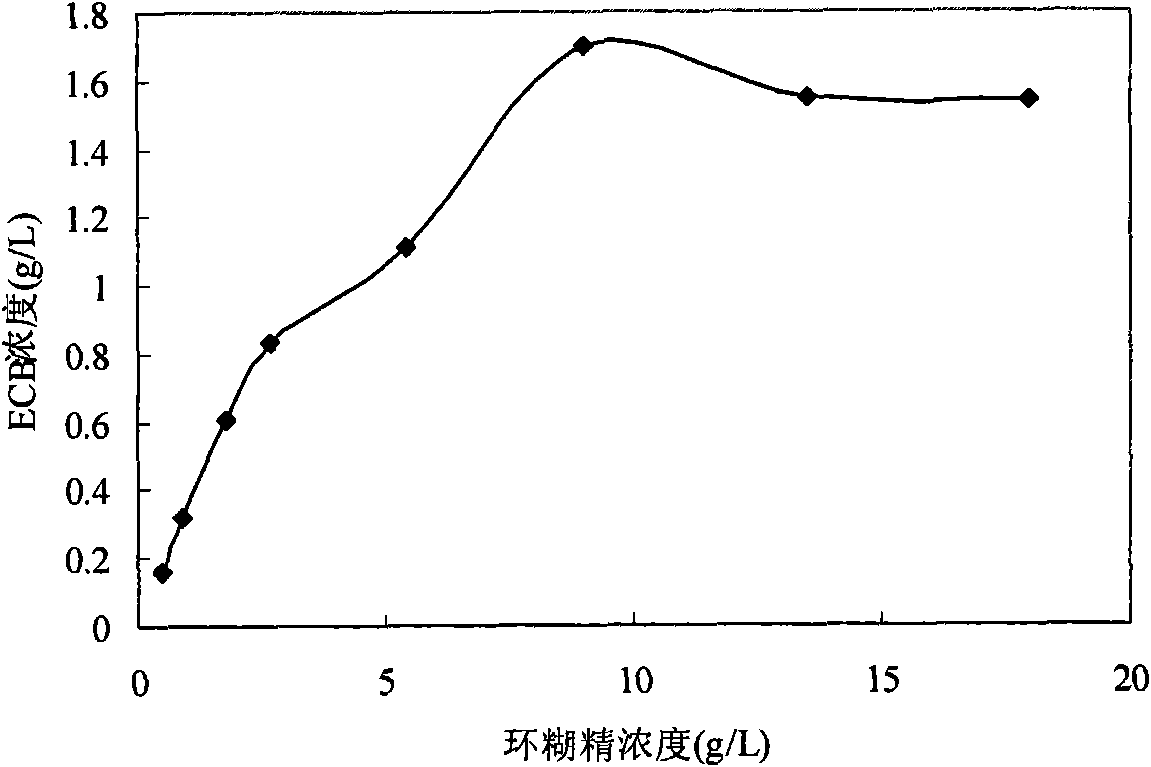
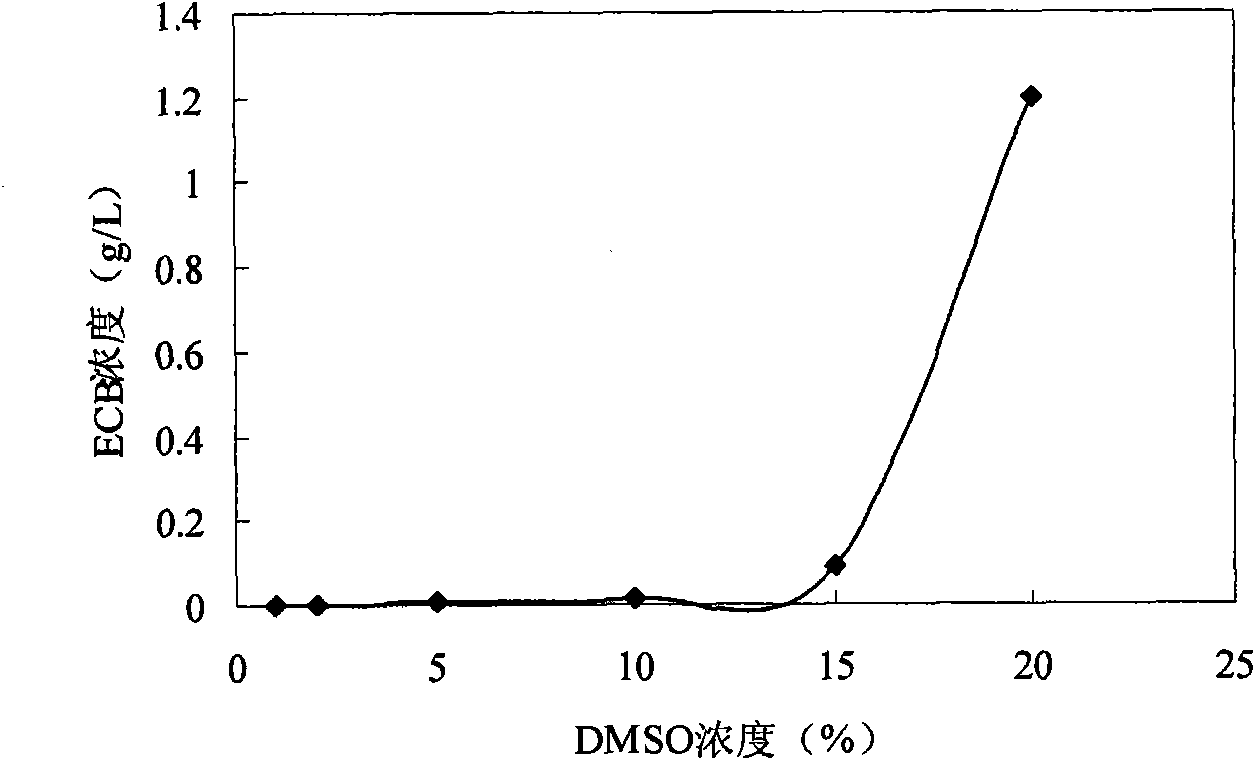
[0050] Example 4: Conversion method of Echinocandin B (ECB) in dimethyl-β-cyclodextrin system
[0051] Dissolve dimethyl-β-cyclodextrin in 50L acetic acid buffer solution with pH 6 concentration of 0.2M, fully dissolve until the solution is clear and transparent, the concentration of cyclodextrin is 12g / L, and grind 100g of ECB into powder Disperse in the above solutions respectively, and stir for 60 min with a magnetic stirrer at 1000 r / min to fully dissolve ECB. The whole-cell catalyst described in Example 1 was added at a concentration equivalent to 5 g dry cells / L, and after transformation at 25° C. for 48 hours, acetic acid was added to adjust the pH to 3 to terminate the transformation.
[0052] Take the transformation solution at 12000r / min, centrifuge for 5min, take the supernatant for HPLC analysis, use ODS C18 (8×100mm), mobile phase A: 3% acetonitrile / 0.5% NH 4 h 2 PO 4 , mobile phase B: 50% acetonitrile / 0.5% NH 4 h 2 PO 4 , Elution condition: 5% B+95% A 3min,...
Embodiment 5
[0053] Example 5: Conversion method of aculeacin A in β-cyclodextrin system
[0054] Dissolve β-cyclodextrin in 50L pH 5 0.1M phosphate buffer solution, fully dissolve until the solution is clear and transparent. Disperse in the above solution, and stir with a magnetic stirrer at 1000r / min for 60min to fully dissolve aculeacin A. The whole-cell catalyst described in Example 2 was added at a concentration equivalent to 4 g dry cells / L, and after transformation at 40° C. for 48 hours, acetic acid was added to adjust the pH to 3 to terminate the transformation.
[0055] Get the conversion solution at 12000r / min, centrifuge for 5min, get the supernatant HPLC analysis, (analysis method is the same as in Example 4), and calculate the conversion rate according to the consumption of aculeacin A to be 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com