Medicinal composition containing echinocandin antifungal agent and its preparing method and its use
A composition and antifungal technology, which can be applied in the directions of antifungal agents, medical preparations of inactive ingredients, pharmaceutical formulations, etc., and can solve the problems of not providing stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0104] Caspofungin formulation preparation
[0105] The preparation process is similar to Comparative Example 1, except that glacial acetic acid is not added during the preparation process, but a phosphate buffer is added, and the pH is adjusted to 5.5-6.5. By changing the concentration of caspofungin acetate and / or adding The concentration of excipient obtains different formulas, and the composition of each formula of composition before freeze-drying is as follows:
[0106]
[0107]
[0108] After each formulation was freeze-dried, the same stability investigation as described in Comparative Example 1 was carried out.
Embodiment 4
[0110] Caspofungin formulation stability
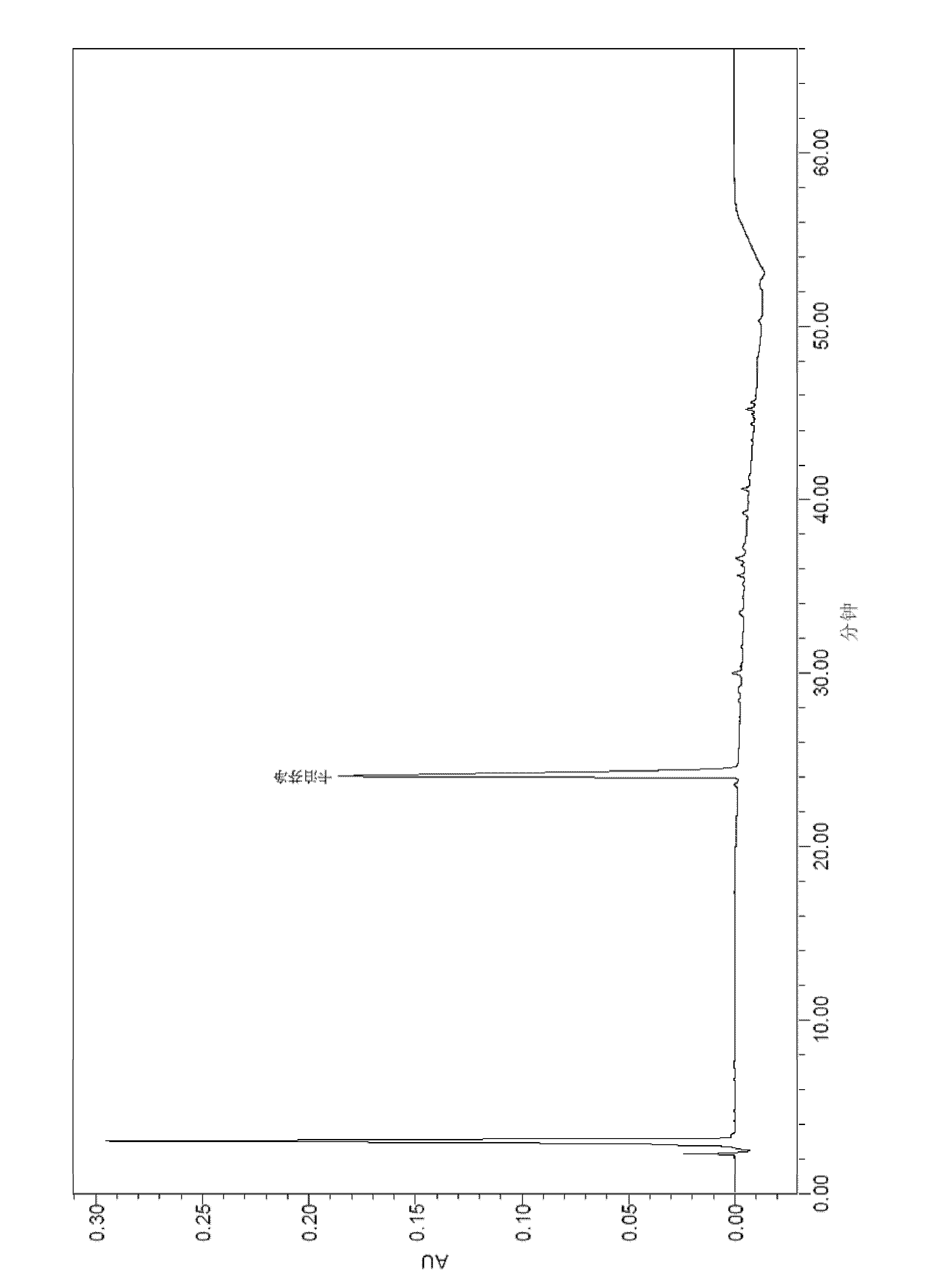
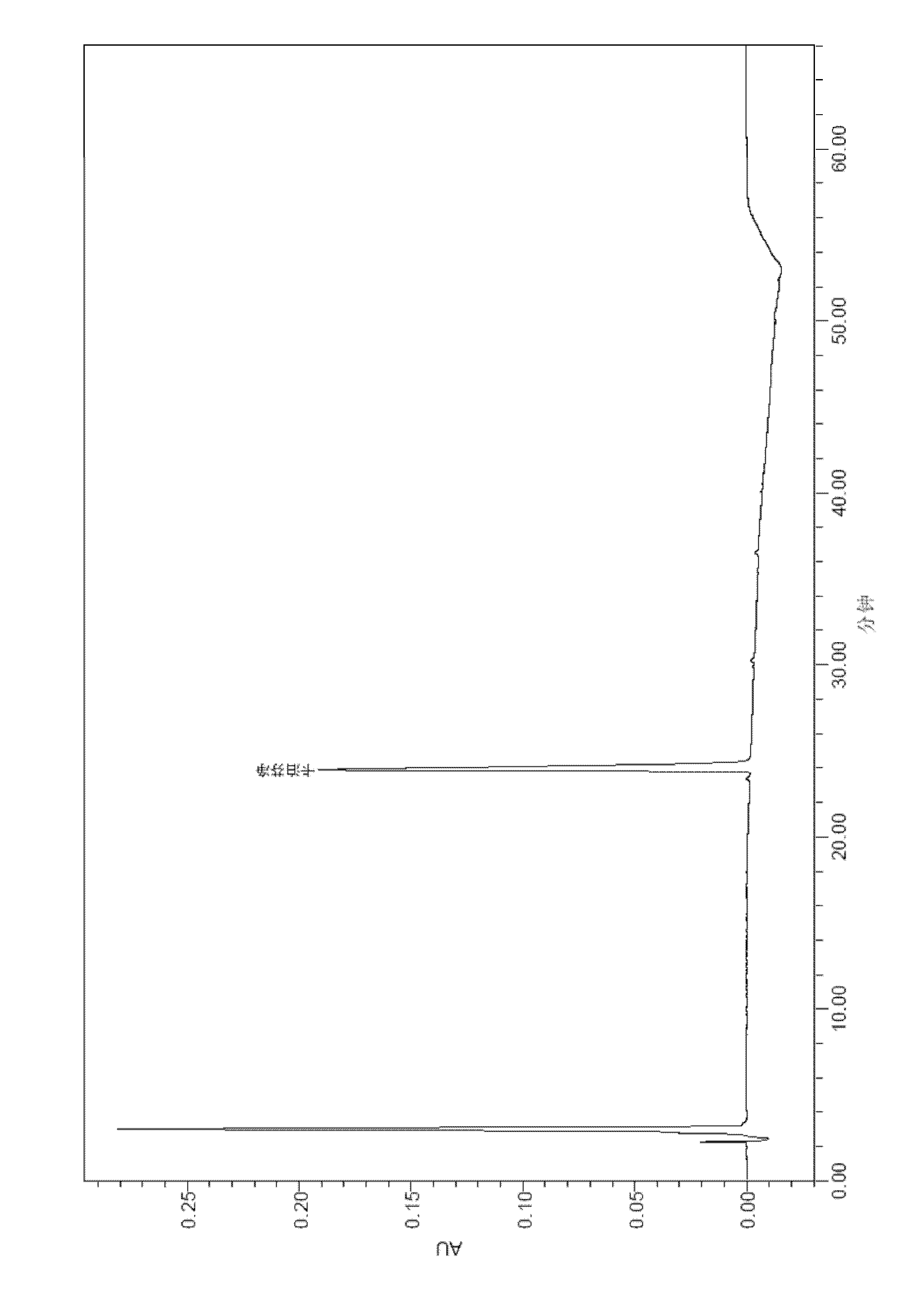
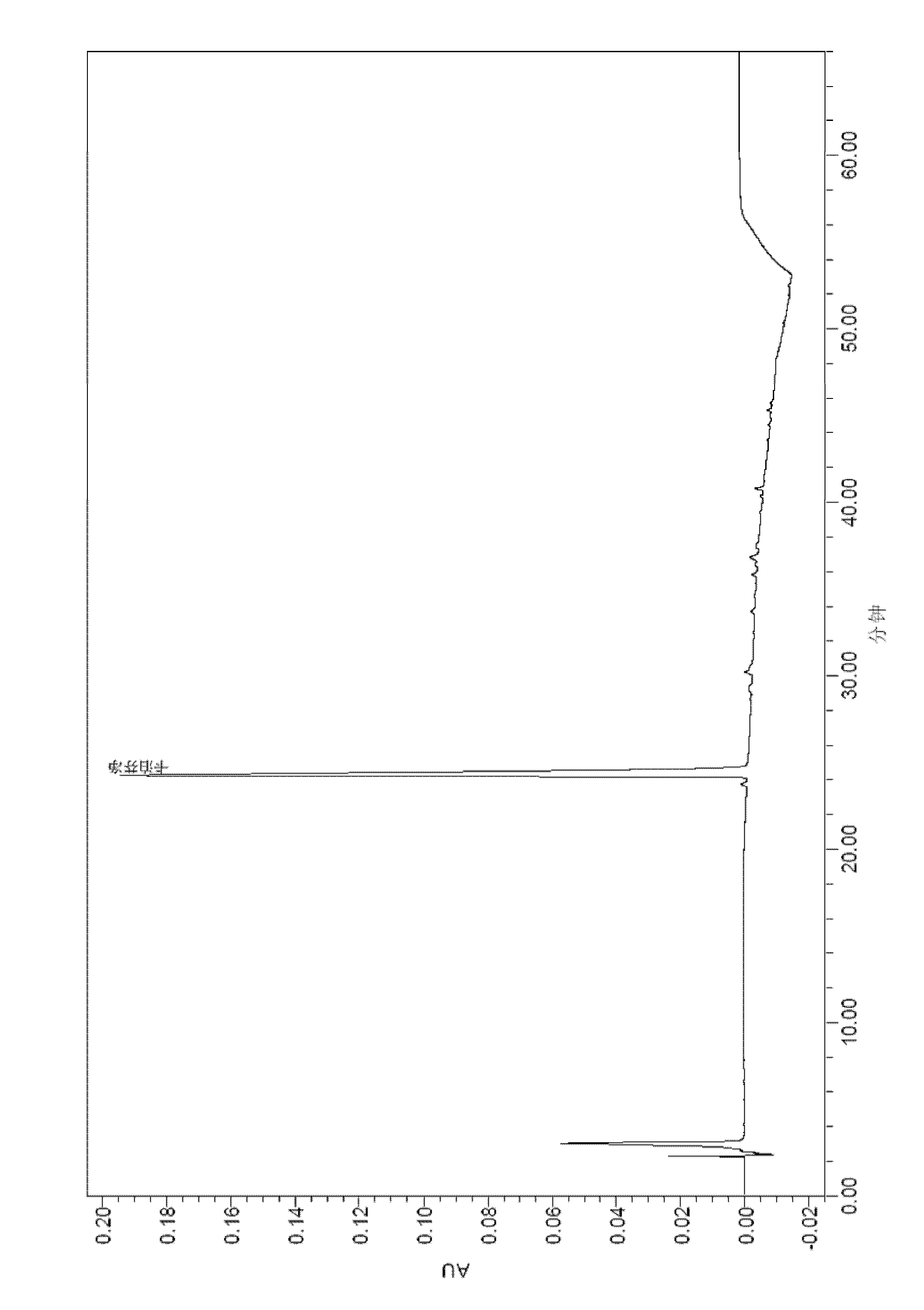
[0111] After the samples of Comparative Examples 1, 2 and Example 3 were tested for stability, the active substances were analyzed by HPLC, and the analysis method used was as above.
[0112] The results of the 40°C stability test are shown in the table below:
[0113]
[0114] The results of the stability test at 30°C are shown in the table below:
[0115]
[0116] The results of the stability test at 2-8°C are shown in the table below:
[0117]
[0118]
[0119] By comparing the above stability test data, it can be seen that each formulation is very stable at 2-8°C, and the content of caspofungin does not decrease significantly after 12 weeks and 24 weeks. The 2-8°C stability data provided by the present invention is carried out on the basis of the raw material medicine produced by our company, wherein the raw material medicine contains process impurities such as RRT0.95 (relative retention time is 0.95), which has a h...
Embodiment 7
[0136] Micafungin formulation preparation
[0137] The preparation process is similar to Comparative Example 5, except that citric acid is not added during the preparation process, but pH adjustment is carried out with phosphate, and different concentrations of micafungin sodium and / or excipients are obtained by changing the concentration of micafungin sodium. The formula, the composition of each formula of the composition before freeze-drying is as follows:
[0138]
[0139]
[0140] After each formula was freeze-dried, the stability test described in Comparative Example 5 was also carried out.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com