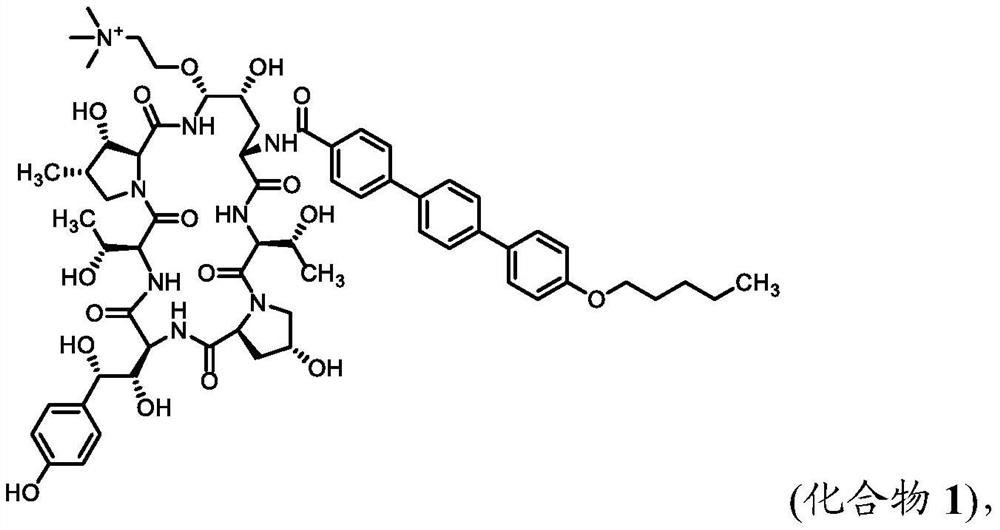
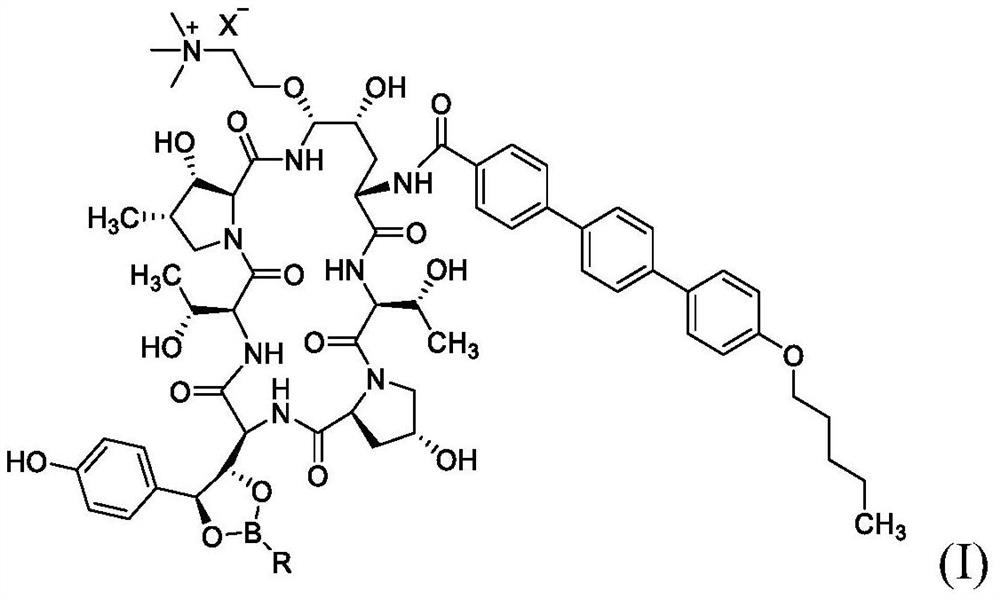
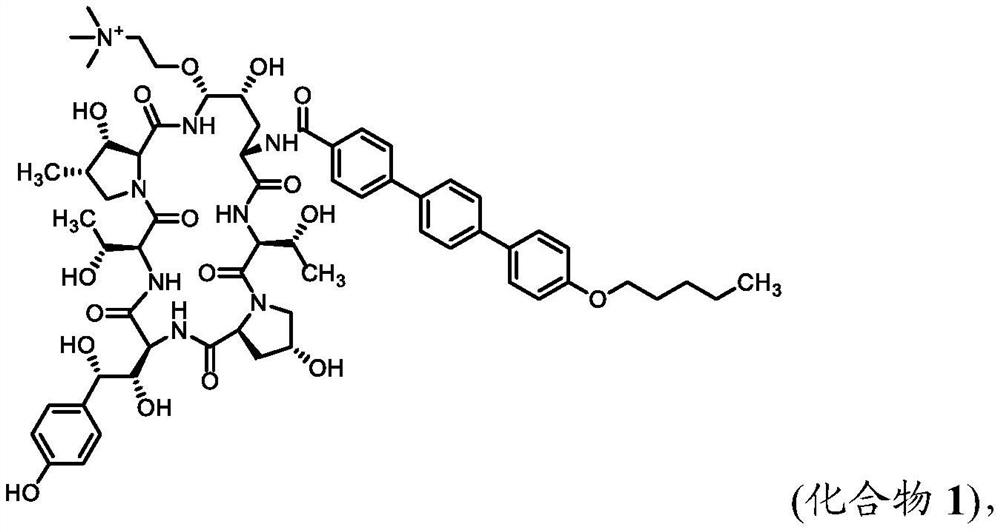
Synthesis of echinocandin antifungal agent
A compound and composition technology, applied in antifungal agents, peptide/protein components, cyclic peptide components, etc., can solve the complex and difficult production of pharmaceutical grade echinocandins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1. Compound 1 was synthesized from the phenylboronic acid ester of Anifungin.
[0098] Anifungin Phenylboronate:
[0099] To a solution of anidulungin (5 g) in tetrahydrofuran (70 mL) was added a solution of phenylboronic acid (0.7 g) in tetrahydrofuran (30 mL). The reaction mixture was stirred at room temperature for 90 minutes. The reaction mixture was concentrated by rotary evaporation. The resulting solid was dissolved in tetrahydrofuran (60 mL) and concentrated by rotary evaporation. The resulting solid was redissolved in tetrahydrofuran (60 mL) and concentrated by rotary evaporation. The resulting solid mixture was redissolved in acetonitrile / tetrahydrofuran (30 mL / 15 mL) and concentrated by rotary evaporation. The resulting anidulungin phenyl borate solid was dried overnight in vacuo.
[0100] Choline Chloride Drying:
[0101] In a round bottom flask, choline chloride (18.6 g) was suspended in acetonitrile (150 mL) and stirred for 4 hours. The susp...
Embodiment 2
[0106] Example 2. Compound 1 was synthesized from 4-(trifluoromethyl)phenylboronate of anidulungin.
[0107] The reaction was performed on a 200 mg scale similar to the method of Example 1 except that the boronic acid was changed to 4-(trifluoromethyl)phenylboronic acid and the reaction time was 24 hours. Results: 63% Compound 1; 7.0% Compound 1 β-diastereomer.
[0108] A second conjugation experiment was performed in which 4-(trifluoromethyl)phenyl boronate was first dissolved in an acetonitrile:TFA mixture, and then a dry solution of choline chloride was added to it. After 2.5 h, the reaction mixture was diluted with water:acetonitrile (70:30) and the pH was adjusted to 2.0 by addition of ammonium hydroxide. Results: 75% Compound 1; 4.8% Compound 1 β-diastereomer.
Embodiment 3
[0109] Example 3. Compound 1 was synthesized from 2,6-dimethylphenyl borate of anidulungin.
[0110] The reaction was performed on a 200 mg scale similar to the method of Example 1 except that the boronic acid was changed to 2,6-dimethylphenylboronic acid. Results: 55% compound 1; 7.4% compound 1 β-diastereomer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com