Echinocandin antifungal agent caspofungin containing medicinal composition, a preparation method and application thereof
A composition and antifungal technology, which is applied in the direction of antifungal agents, medical preparations with non-active ingredients, pharmaceutical formulations, etc., and can solve problems such as not providing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0088] Caspofungin formulation preparation
[0089] The preparation process is as follows: first dissolve the stabilizer sucrose in water or in a solution containing an optional pH regulator, then add the compound of formula I or its pharmaceutically acceptable salt to dissolve it, set the volume to a certain volume, and then Lyophilization was carried out.
[0090] Different formulations are obtained by varying the concentration of caspofungin and / or the concentration of sucrose, and the pH or concentration of the pH adjuster. The composition of each formulation of the composition before lyophilization is as follows:
[0091]
[0092]
[0093] After each formulation was freeze-dried, the same stability investigation as described in Comparative Example 1 was carried out.
Embodiment 3
[0095] Caspofungin formulation stability
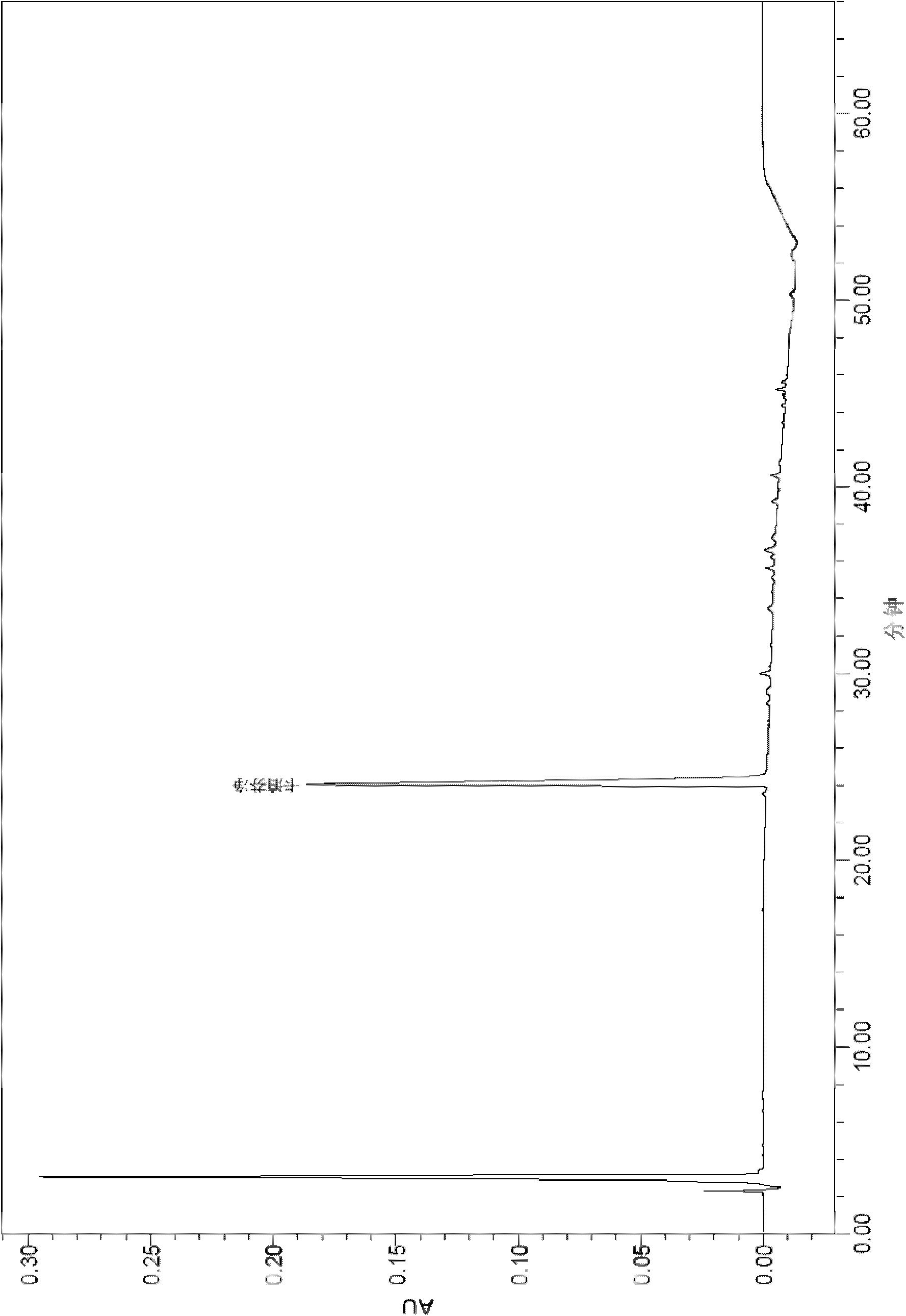
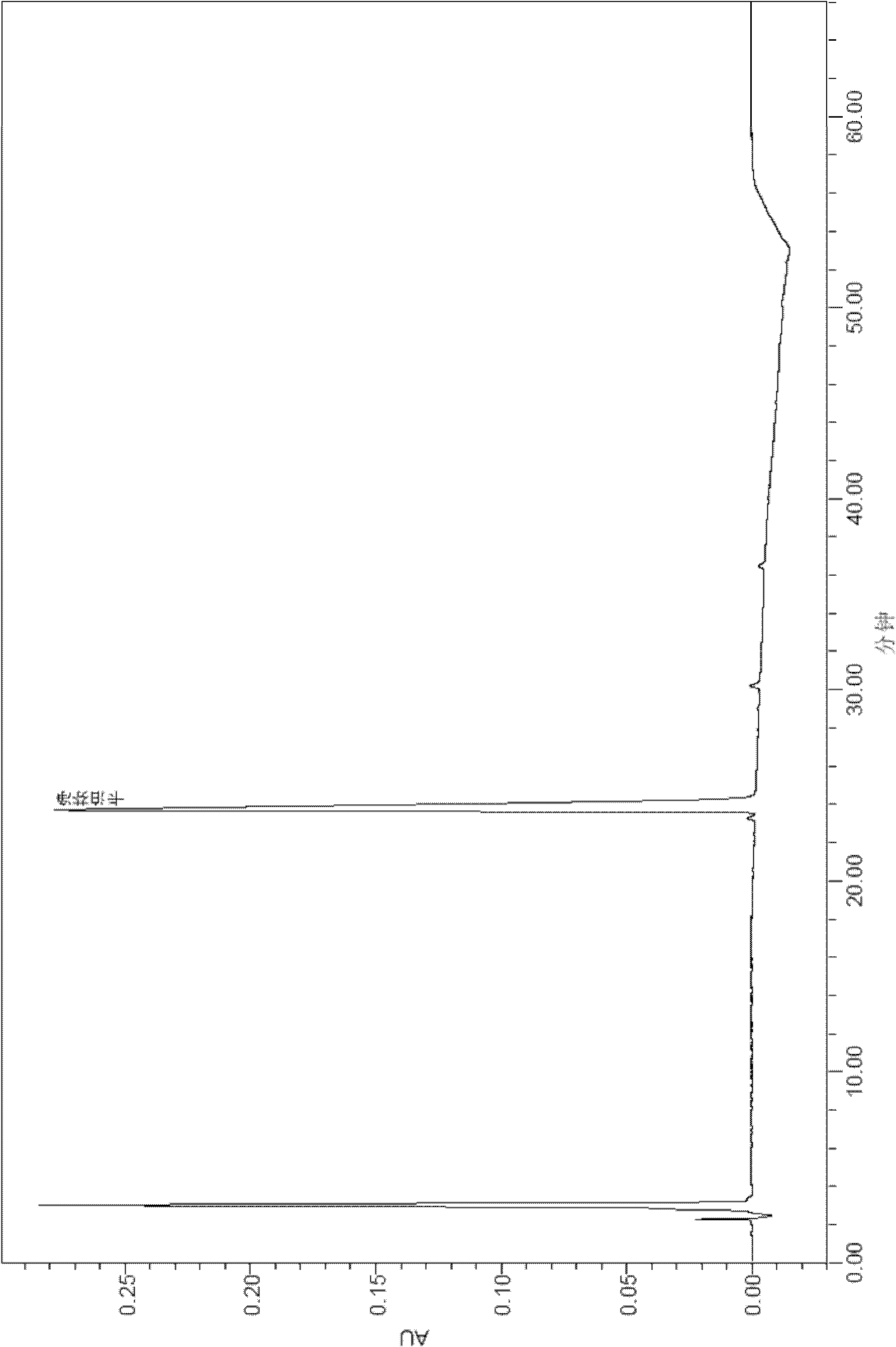
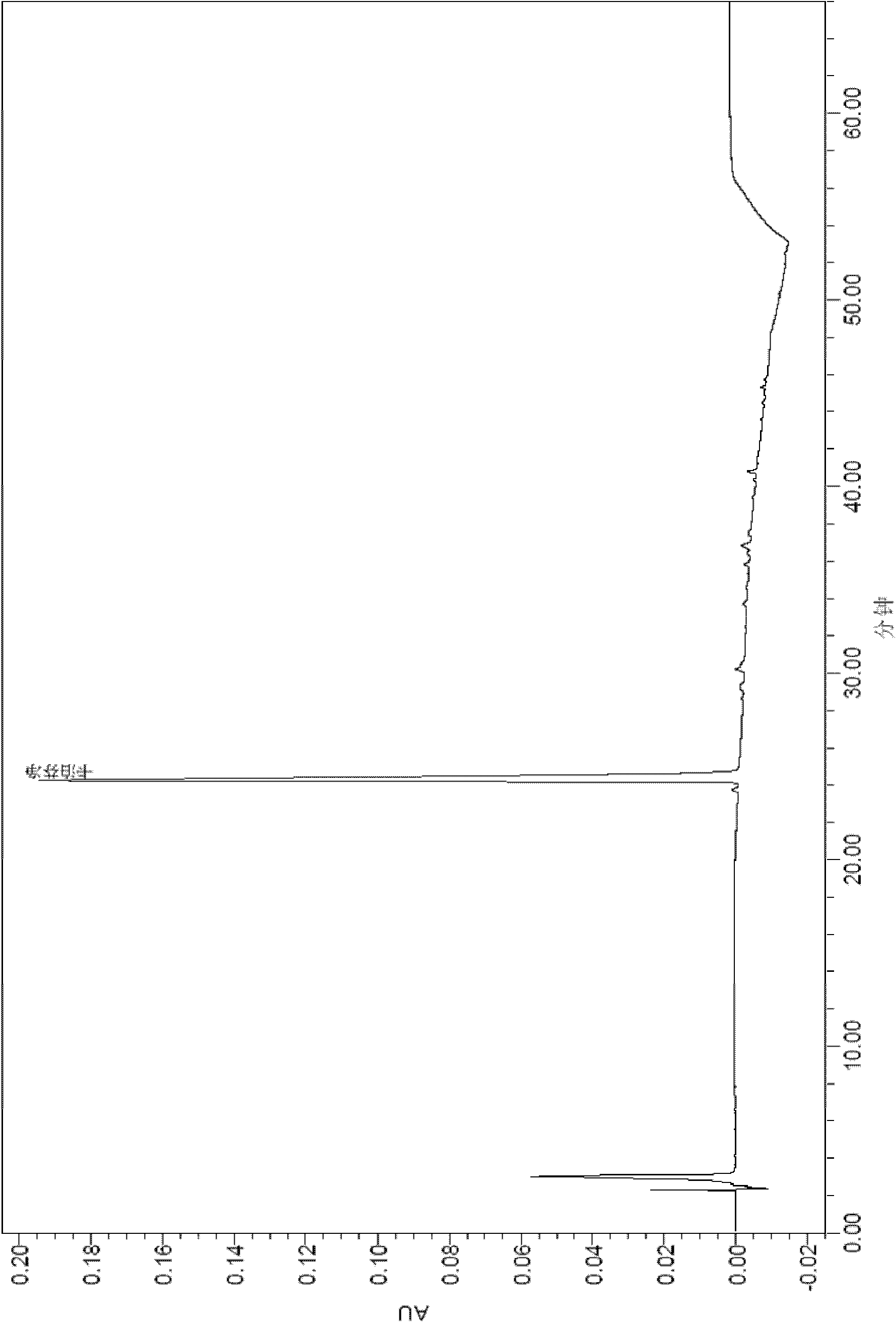
[0096] After the samples of Comparative Example 1 and Example 2 were tested for stability, the active substances were analyzed by HPLC.
[0097] The results of the 40°C stability test are shown in the table below:
[0098]
[0099] The results of the stability test at 30°C are shown in the table below:
[0100]
[0101] The results of the stability test at 2-8°C are shown in the table below:
[0102]
[0103]
[0104] By comparing the above stability test data, it can be seen that each formulation is very stable at 2-8°C, and the content of caspofungin does not decrease significantly after 12 weeks and 24 weeks. The 2-8°C stability data provided by the present invention is carried out on the basis of the raw material medicine produced by our company, wherein the raw material medicine contains process impurities such as RRT0.95 (relative retention time is 0.95), which has a high degree of stability in stability. There wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com