Echinocandin antifungal pharmaceutical composition sustained release microsphere preparation and preparation method thereof
A technology for sustained-release microsphere preparations and antifungal drugs, which can be used in antifungal agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve problems such as potential changes and visual disturbances.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
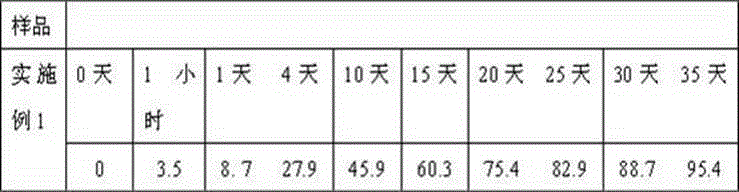
Embodiment 1
[0018] Weigh 10g caspofungin acetate, 10g amphotericin B, 20g gelatin, 20g glycerin, 20ml 0.5% polyvinyl alcohol solution, 20ml absolute ethanol and dissolve in distilled water to obtain the internal aqueous phase; weigh 800g polylactide Dissolve in dichloromethane to get oily phase. First move the water phase into the oil phase, place it on an emulsifying disperser at 30000 rpm at room temperature, and homogenize for 30 seconds, then transfer the resulting W / O emulsion to an aqueous solution of polyvinyl alcohol and place it on the emulsifying disperser at 5000 rpm. Rotate and homogenize for 1 minute to obtain W / O / W double emulsion. Stir at low speed at 500 rpm for 2 hours at room temperature, remove organic solvent, ultracentrifuge, collect the resulting microspheres, wash with distilled water several times, and then centrifuge to collect , Adding 90g sorbitol, freeze-drying, and distributing into a echinocandin antifungal sustained-release microsphere preparation with an act...
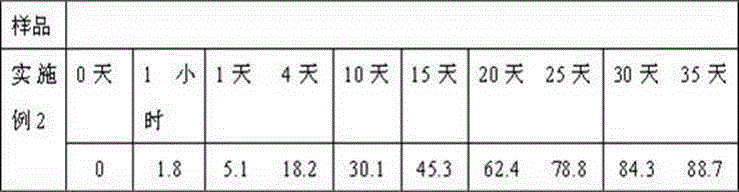
Embodiment 2
[0027] Weigh 60g caspofungin acetate, 20g fluconazole, 30g gelatin, 30g glycerin, 20ml 0.5% polyvinyl alcohol solution, 30ml propylene glycol and dissolve in distilled water to obtain the internal water phase; weigh 720g polyglycolide to dissolve In dichloromethane, the oily phase is obtained. First move the water phase into the oil phase, place it on an emulsification disperser at a rotation speed of 30,000 rpm, and homogenize for 30 seconds, then transfer the obtained W / O type emulsion to 50 ml of purified water, and place it on the emulsification disperser at a rotation speed of 5000 rpm. Homogenize the milk for 1 minute to obtain a W / O / W double emulsion, place it on a mechanical stirrer, stir at 500 rpm at low speed for 2 hours at room temperature, centrifuge, collect the resulting microspheres, wash with distilled water several times, and then centrifuge to collect , Adding 70g of mannitol, freeze-drying, and distributing it into a echinocandin antifungal sustained-release...
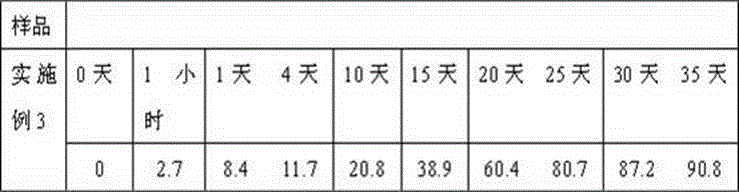
Embodiment 3
[0036] Weigh 110g caspofungin acetate, 30g itraconazole, 40g gelatin, 40g glycerin, 20ml 0.5% polyvinyl alcohol solution, 40ml absolute ethanol and dissolve in distilled water to obtain the internal aqueous phase; weigh 700g polylactide -Glycolide is dissolved in dichloromethane to obtain an oil phase. Prepare 50ml of polyvinyl alcohol solution with a concentration of 5%. First move the water phase into the oil phase, place it on an emulsifying disperser at room temperature at 30000 rpm and homogenize for 30 seconds, then transfer the resulting W / O emulsion to 50 ml of a 5% polyvinyl alcohol solution and place On the emulsifying and dispersing machine at 5000 rpm, the milk is homogenized for 1 minute to obtain W / O / W double emulsion, placed on a mechanical stirrer, stirred at 500 rpm at low speed for 2 hours at room temperature, centrifuged, collected the resulting microspheres, and used distilled water Wash it several times, then collect by centrifugation, add 40g of mannitol,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com