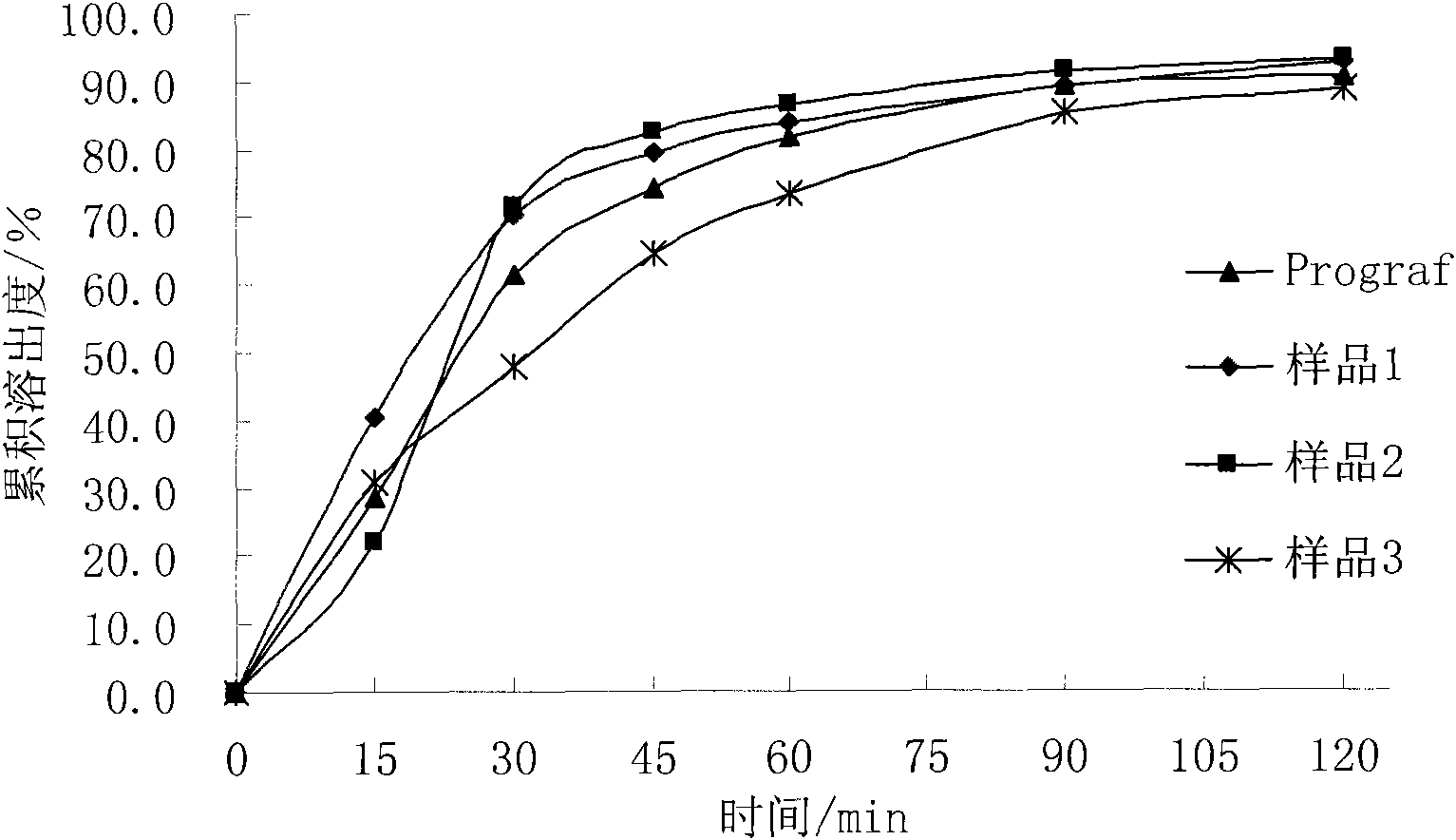
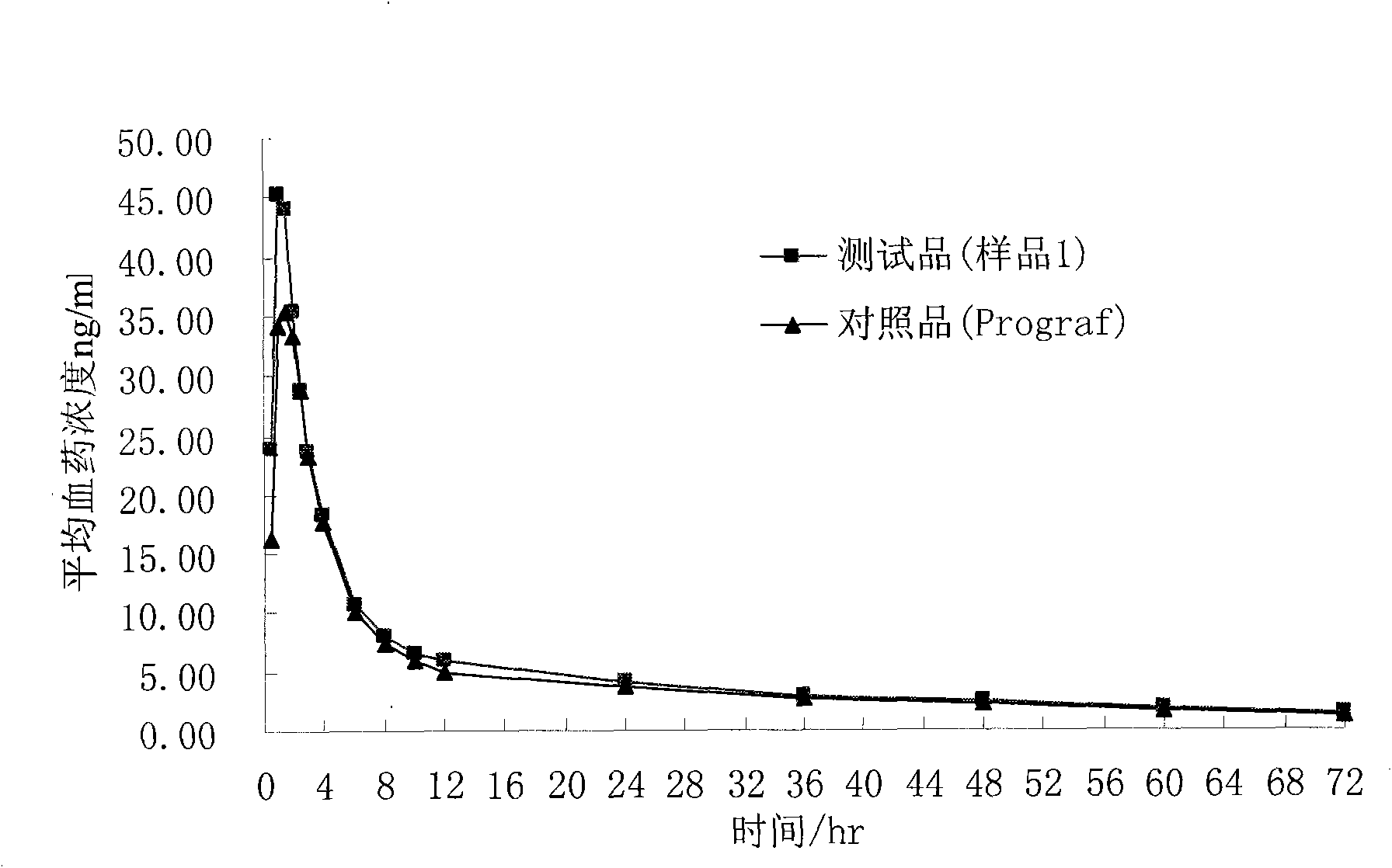
Composition containing water-insoluble high-activity drug and preparation method thereof
A composition and high-activity technology, applied in the field of medicine, can solve problems such as uneven mixing, and achieve the effects of good drug dissolution, simple process, oral absorption and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Capsules and tablets with FK506 as active ingredient
[0030] FK506 1g
[0031] HPMC 1g
[0032] Lactose 63g
[0033] Dissolve FK506 in 10mL ethanol, add HPMC to fully disperse, add 15mL dichloromethane to dissolve it. Spray the above solution evenly on the surface of the lactose granules in the fluidized bed, the blast pressure is 0.1MPa, the spraying speed is 1mL / min, the spraying pressure is 0.05MPa, and the material is dried for 5min after the drug application is completed.
[0034] The prepared composition can be added with 66.5g of starch, 7g of croscarmellose sodium and 1.5g of magnesium stearate, mixed evenly and filled into No. 4 capsules to make capsules with a marked amount of 1mg, namely the sample 1.
[0035] The prepared composition can be added with 160g of compressible starch, 60g of microcrystalline cellulose, 14g of croscarmellose sodium and 1g of magnesium stearate, mixed evenly and pressed into tablets, the tablet weight is 300mg, a...
Embodiment 2
[0036] Embodiment 2: Capsules, tablets, dry suspensions with FK506 as active ingredient
[0037] FK506 1g
[0038] PVP 0.5g
[0039] HPMC 1.5g
[0041] Dissolve FK506 in 12mL methanol, add PVP to fully dissolve, then add HPMC to fully disperse, add 18mL chloroform to dissolve. Spray the above solution evenly on the surface of the sucrose pellets in the fluidized bed, the blast pressure is 0.15MPa, the spraying speed is 1mL / min, the spraying pressure is 0.05MPa, and the material is dried for 5min after the drug application is completed.
[0042] The prepared composition can be added with 75g blank ball core, mixed evenly and filled into No. 4 capsules to make capsules with a marked amount of 1 mg, i.e. sample 2.
[0043] The prepared composition can be added with 235g of blank ball cores, mixed evenly, and pressed into tablets with a tablet weight of 300mg and a marked amount of 1mg.
[0044] The prepared composition can be added with 75g of bla...
Embodiment 3
[0045] Embodiment 3: Capsules, tablets, dry suspensions with FK506 as active ingredient
[0046] FK506 1g
[0047] HPC 5g
[0048] Eudragit 2g
[0049] Microcrystalline Cellulose Ball Core 57g
[0050] Dissolve FK506 in 25mL ethanol, add HPC and Eudragit to fully dissolve. Spray the above solution evenly on the surface of the microcrystalline cellulose pellet core in the fluidized bed, the blast pressure is 0.15MPa, the liquid spray speed is 1mL / min, the liquid spray pressure is 0.05MPa, and the material is dried for 5min after the drug application is completed.
[0051] The prepared composition can be added with 75g blank ball core, mixed evenly and filled into No. 4 capsules to make capsules with a marked amount of 1 mg, i.e. sample 3.
[0052] The prepared composition can be added with 235g of blank ball cores, mixed evenly, and pressed into tablets with a tablet weight of 300mg and a marked amount of 1mg.
[0053] The prepared composition can be added with 75g of blan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com