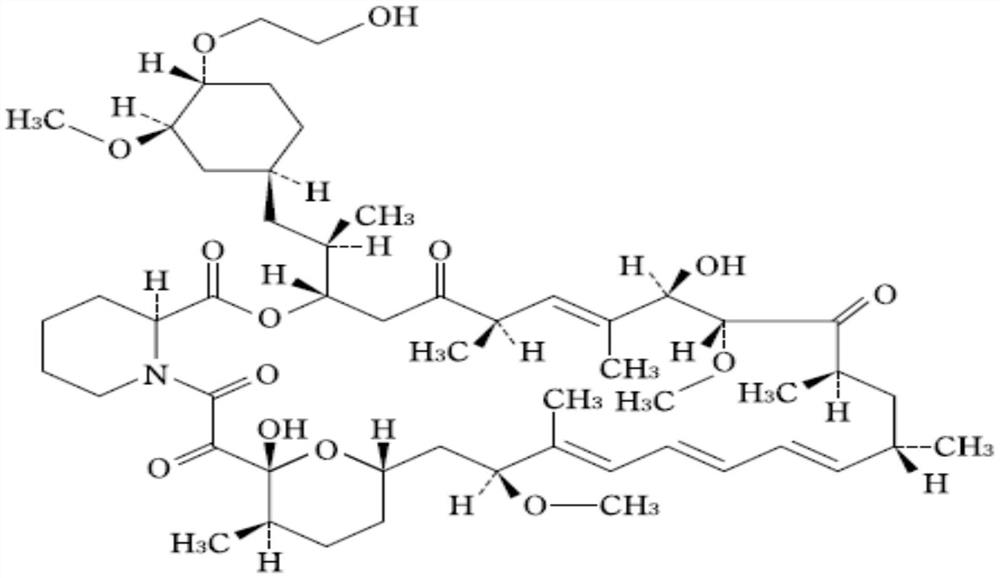
Stable 40-O-(2-ethoxyl)-rapamycin tablet and preparation method thereof
The technology of rapamycin tablets and rapamycin, which is applied in the field of medicine, can solve problems such as hidden safety hazards, multiple impurities, and poor stability, and achieve the effects of reducing equipment requirements, simplifying process steps, and reducing hygroscopicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
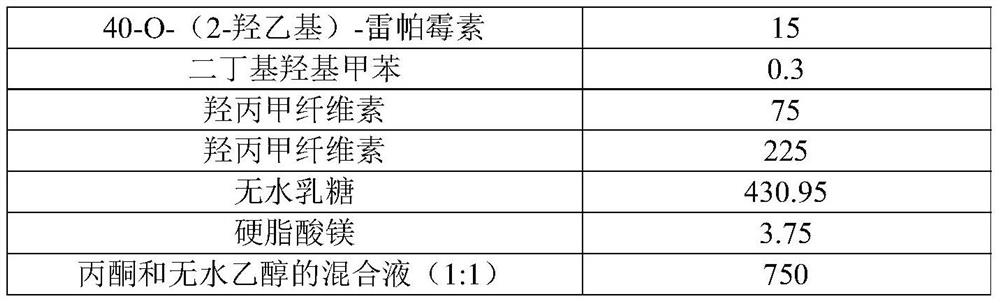
[0038] Table 1
[0039] composition batch / g 40-O-(2-hydroxyethyl)-rapamycin 15 2,6-Di-tert-butyl-4-methylphenol 0.3 Hypromellose 60 Hypromellose 75 anhydrous lactose 595.95 Magnesium stearate 3.75 Acetone, absolute ethanol mixture (1:1) 750
[0040] Preparation Process:
[0041] Add 40-O-(2-hydroxyethyl)-rapamycin, 2,6-di-tert-butyl-4-methylphenol and part of hypromellose into absolute ethanol and acetone to dissolve, spray Add the remaining amount of hypromellose and part of the anhydrous lactose into the fluidized bed granulation to obtain dry granules for granulation, and uniformly mix with the remaining amount of anhydrous lactose and magnesium stearate, and then tableting can be obtained.
Embodiment 2
[0043] Table 2
[0044] composition batch / g 40-O-(2-hydroxyethyl)-rapamycin 15 2,6-Di-tert-butyl-4-methylphenol 0.3 Hypromellose 30 Hypromellose 105 anhydrous lactose 595.95 Magnesium stearate 3.75 Acetone, absolute ethanol mixture (1:1) 1200
[0045] Preparation Process:
[0046]Add 40-O-(2-hydroxyethyl)-rapamycin, 2,6-di-tert-butyl-4-methylphenol, and hypromellose into absolute ethanol and acetone to dissolve, spray into The hypromellose is subjected to fluidized bed granulation, and the obtained dry granules are granulated, mixed with anhydrous lactose and magnesium stearate, and pressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com