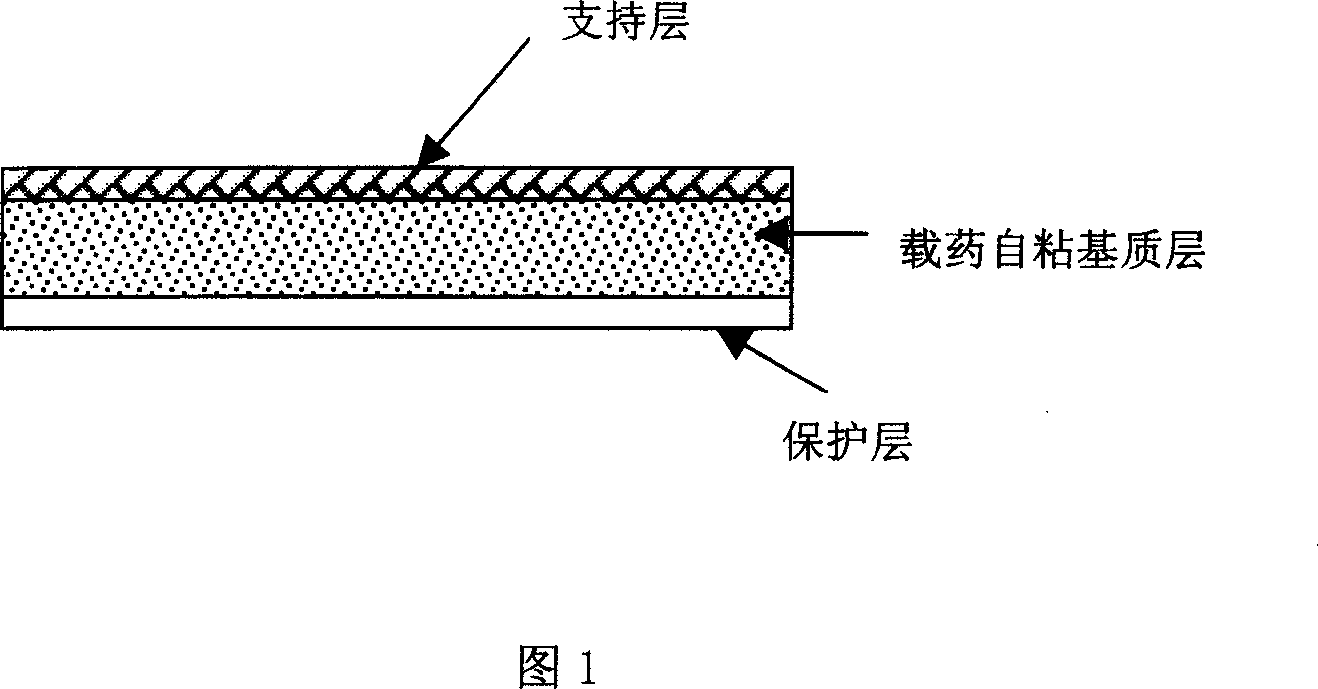
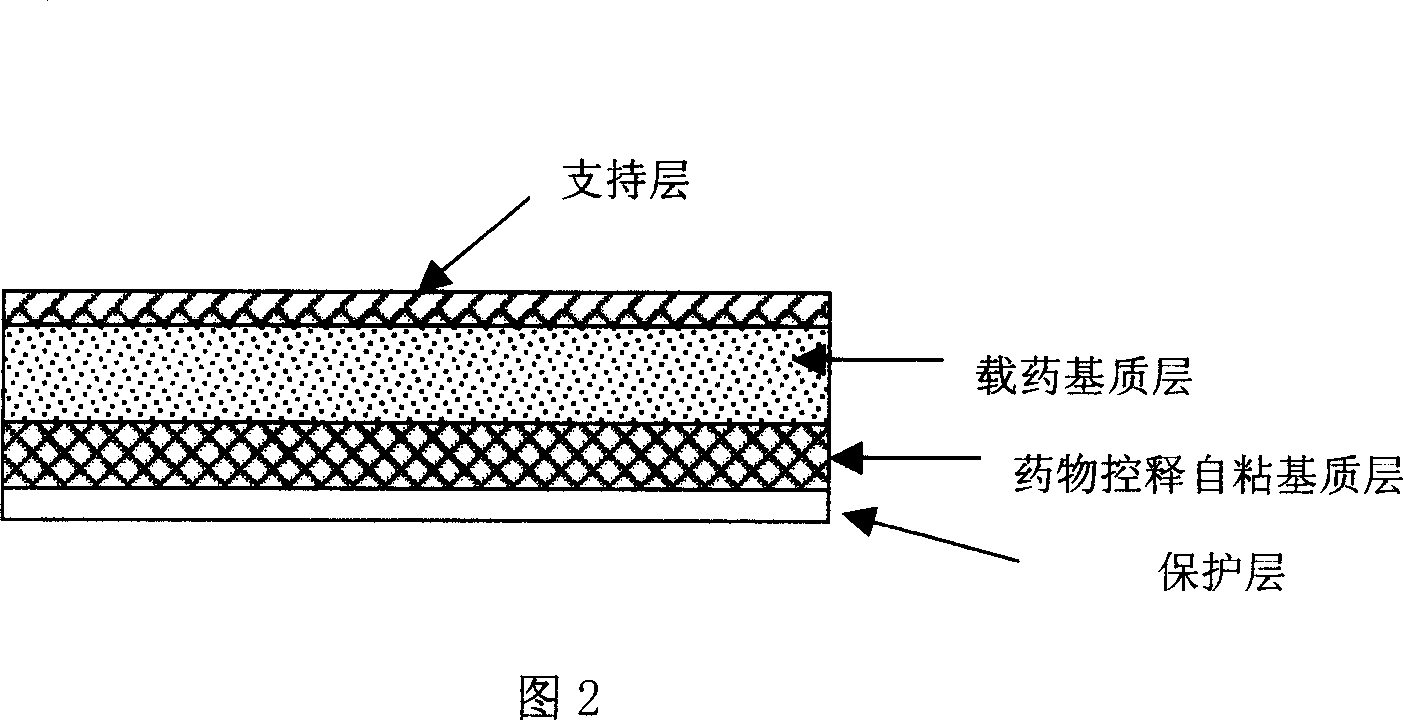
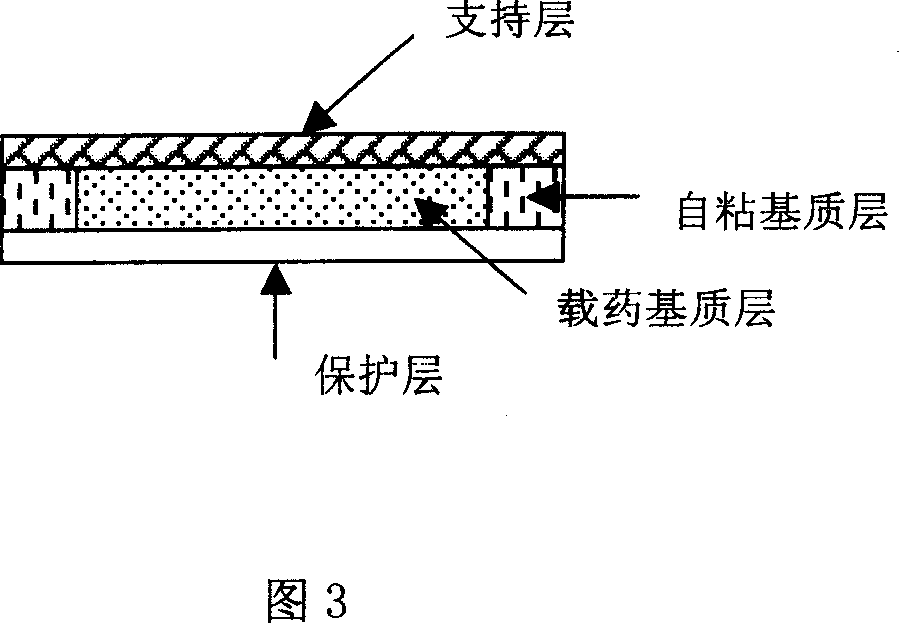
Rasagiline transparent patch for curing and preventing neurological diseases and the preparing method thereof
A technique for nervous system diseases and patches, which is applied in the field of rasagiline transdermal drug patches and its preparation, protective foils or membranes, which can solve the problems of unsatisfactory transdermal penetration effects and inconvenient administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Single-layer polyacrylate matrix patch containing rasagiline, with oleic acid as skin penetration promoting agent
[0061] Add 50 grams of 50% (w / w) Eudragit E100 solution in chloroform to a solution of 250 grams of fully swollen polyacrylate adhesive in water; add 50 grams of oleic acid (that is, a low amount of oleic acid) After that, stir to make it even.
[0062] Dissolve 50 grams of rasagiline in 200 ml of absolute ethanol at 50°C to 80°C, and then add it to the stirring solution above. Slowly add 1mol / l NaOH aqueous solution and stir while adding to make the pH value 7.5. After stirring evenly, apply it to the medical non-woven fabric with an appropriate scraper, and adjust the thickness of the wet film to make it in the The weight was 60 g / m2 after drying at 100°C for 60 minutes.
[0063] The dried base film was coated with a 23 μm thick polyester film; each patch was then die-cut into final sheets.
[0064] The amount of oleic acid added can be 150...
Embodiment 2
[0071] Example 2: A single-layer polyvinylpyrrolidone matrix patch containing rasagiline, using isopropyl myristate as a skin penetration promoting agent
[0072] 20 grams of sodium hydroxide and 15 grams of phosphoric acid are added to 500 grams of 25% (w / w) polyvinylpyrrolidone ethanol solution, and after stirring for 1 hour at 600 rpm, add 100 grams of colloid in batches successively Silicon dioxide, add 80 grams of rasagiline and 20 grams of isopropyl myristate (low amount of isopropyl myristate) in batches, continue stirring while slowly adding 1 mol / l NaOH aqueous solution to make the pH value 7.5 , after stirring for at least 1 hour to make it uniform, apply it to the medical non-woven fabric with an appropriate spatula, adjust the thickness of this wet film, and make it dry at 80°C for 60 minutes and have a weight of 60 g / m2 .
[0073] The dried base film was coated with a 23 μm thick polyester film; each patch was then die-cut into final sheets.
[0074] The amount ...
Embodiment 3
[0081] Example 3: Multi-layer polyisobutylene matrix patch containing rasagiline, using laurozozone as skin penetration promoting agent
[0082] Dissolve 50g of rasagiline in 200ml of absolute ethanol at 50°C~80°C, then add it to 74% silicone-containing polymer (40g BioPSA 7-4201+40g BioPSA 7-4301) In the heptane solution, after adding 100 grams of petroleum ether and 50 grams of laurozone (that is, a low amount of laurozone), the mixture was stirred for 1 hour at a speed of 600 rpm, while stirring Slowly add 1mol / l NaOH aqueous solution to make the pH value 7.5. After obtaining a uniform dispersion, apply it to the medical non-woven fabric with an appropriate scraper, adjust the thickness of the wet film, and make it dry at 60°C The weight after drying for 60 minutes was 60 g / m2. 150g, 250g
[0083] The dried base film was coated with a 23 μm thick polyester film; each patch was then die-cut into final sheets.
[0084] The amount of laurocapram added can be 150 grams (that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com