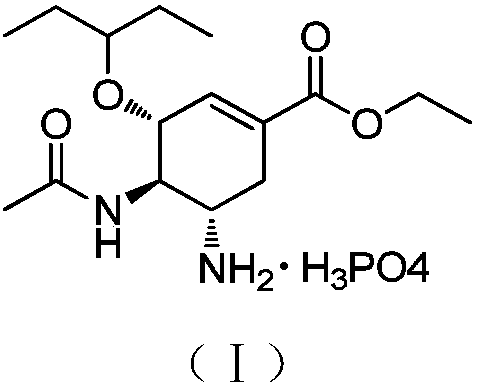
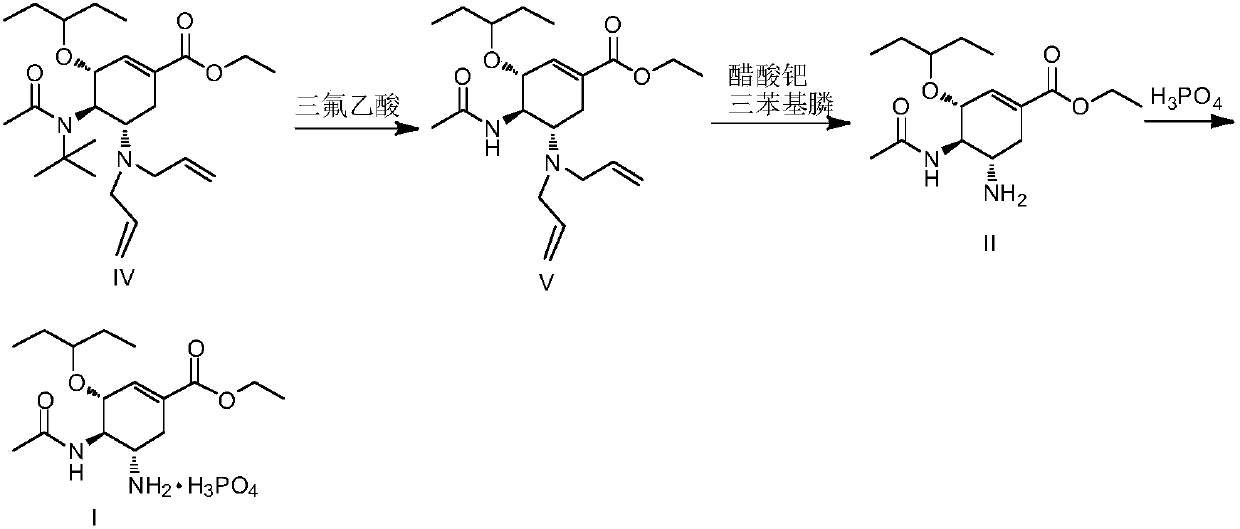
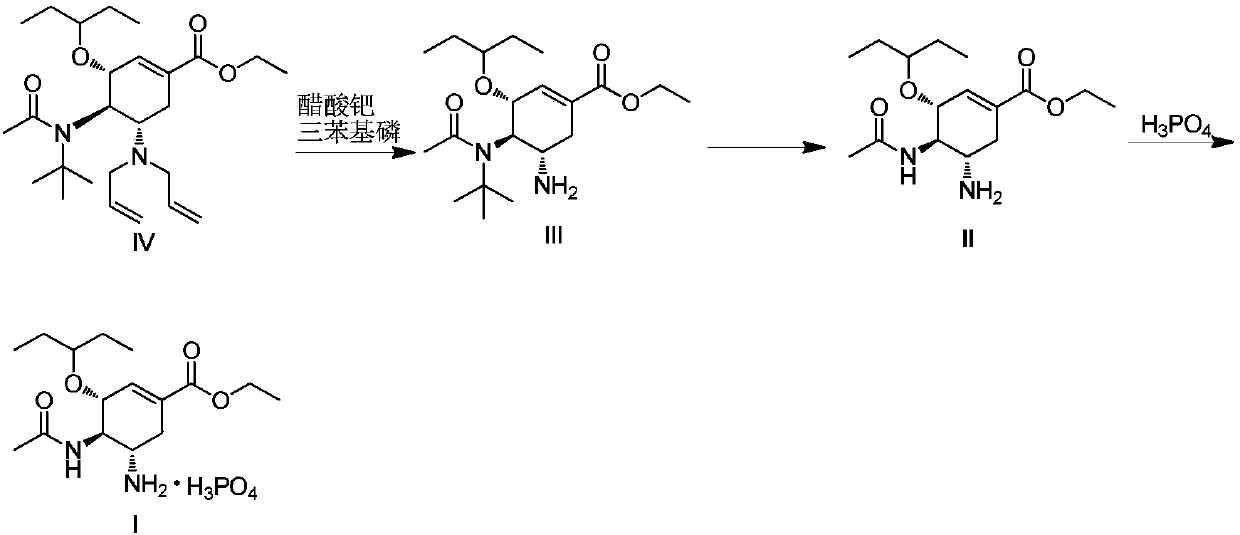
Method for preparing oseltamivir phosphate
A technology of oseltamivir and palladium acetate, which is applied in the field of preparation of oseltamivir phosphate, can solve the problems of excessive residues of heavy metal elements and impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A comparative example of oseltamivir phosphate was prepared using the method disclosed in CN1759093.
[0022]
[0023] Weigh 200 g of the compound represented by formula (IV) and dissolve it in 300 ml of dichloromethane, add it dropwise to 800 ml of trifluoroacetic acid, and react for 1 h at a temperature controlled to ≤50°C. The end point was monitored by TLC, the solvent was concentrated under reduced pressure, and toluene was added to dry it up three times. Add 800ml of toluene and 800ml of purified water to the concentrated solution, stir vigorously for 30min, and separate the layers. The organic layer is quenched with 400ml×2 purified water, and the aqueous layers are combined. The aqueous layer was quenched with 200ml×2 toluene. Add 800ml of dichloromethane to the aqueous layer, and use saturated Na 2 CO 3 The aqueous solution was adjusted to pH=9.0. Let stand to layer. The aqueous layer was extracted with 400ml×3 dichloromethane. The dichloromethane laye...
Embodiment 2
[0031]
[0032] Under nitrogen protection, in a 2000ml reaction flask, add the compound represented by formula (IV) (179.5g, 0.40mol), absolute ethanol 776g, N,N-dimethylbarbituric acid (75.0g, 0.48mol) , palladium acetate (0.90g, 0.004mol), and triphenylphosphine (4.20g, 0.016mol) were added, and the temperature was controlled at 20-25°C, and the reaction was stirred for 2h. Cool down to 10-15°C, keep warm for 30 minutes, filter with suction, and concentrate the filtrate to obtain the crude product of formula (Ⅲ).
[0033] The above crude product of formula (III) was added dropwise into 800ml of trifluoroacetic acid, and reacted at a temperature of 35-40°C for 1h. The temperature of the reaction solution was controlled to be ≤50°C, the solvent was concentrated under reduced pressure, and toluene was added to dry it three times. Add 800ml of toluene and 800ml of purified water to the concentrated solution, stir vigorously for 30min, and separate the layers. The organic lay...
Embodiment 3
[0040] Reaction formula is with embodiment 2.
[0041] Under nitrogen protection, in a 1000ml reaction flask, add the compound represented by formula (IV) (44.9g, 0.10mol), absolute ethanol 194g, N,N-dimethylbarbituric acid (18.8g, 0.12mol) , palladium acetate (0.23g, 0.001mol), triphenylphosphine (1.05g, 0.004mol), after the addition is completed, the temperature is controlled at 20-25°C, and the reaction is stirred for 2h. Cool down to 10-15°C, keep warm for 30 minutes, filter with suction, and concentrate the filtrate to obtain the crude product of formula (Ⅲ).
[0042]The crude product of the above formula (III) was dissolved in 45ml of dichloromethane, added dropwise into 200ml of trifluoroacetic acid, and reacted at a temperature of 35-40°C for 2h. The temperature of the reaction solution was controlled to be ≤50°C, the solvent was concentrated under reduced pressure, and toluene was added to dry it three times. Add 200ml of toluene and 200ml of purified water to the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com