Analysis method for separating and detecting oseltamivir phosphate intermediate and impurities thereof
A technology of oseltamivir phosphate and analysis method, applied in the field of analytical chemistry, to achieve the effects of short detection time, guaranteed specificity, and optimized operating parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
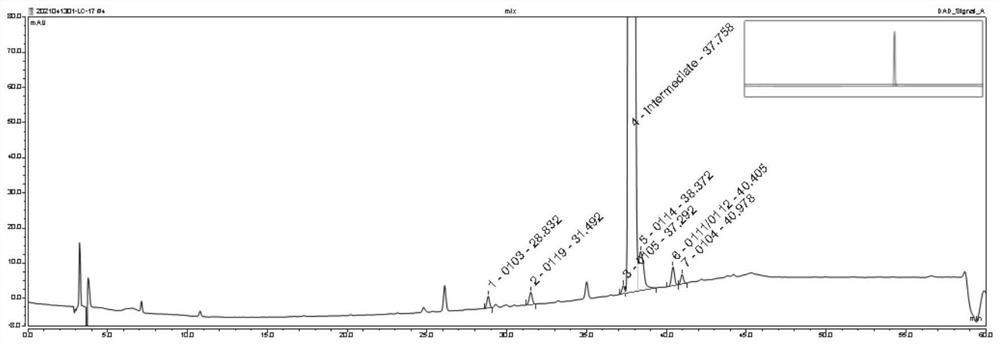
[0055]Preparation of system test solution: Take an appropriate amount of oseltamivir phosphate intermediate and the above impurities 0103, 0104, 0105, 0109, 0111, 0112, 0114, dissolve and dilute with acetonitrile-water (30:70) to obtain oseltamivir phosphate The concentration of the intermediate is 1.0mg / ml, and the system test solution of each impurity is 2μg / ml;
[0056] Preparation of impurity localization solution: Dissolve and dilute appropriate amounts of the above impurities 0103, 0104, 0105, 0109, 0111, 0112, and 0114 with a solvent (acetonitrile-water (30:70)) to obtain a localization solution with a concentration of each impurity of 0.1 mg / ml. solution;
[0057] Use reversed-phase high-performance liquid chromatography to detect, detection conditions:
[0058] Column: Column model: Thermo scientific Hypersil GOLD TM ; Chromatographic column filler: octadecylsilane bonded silica gel; the specifications of the chromatographic column: the inner diameter is 4.6mm, the ...
Embodiment 2
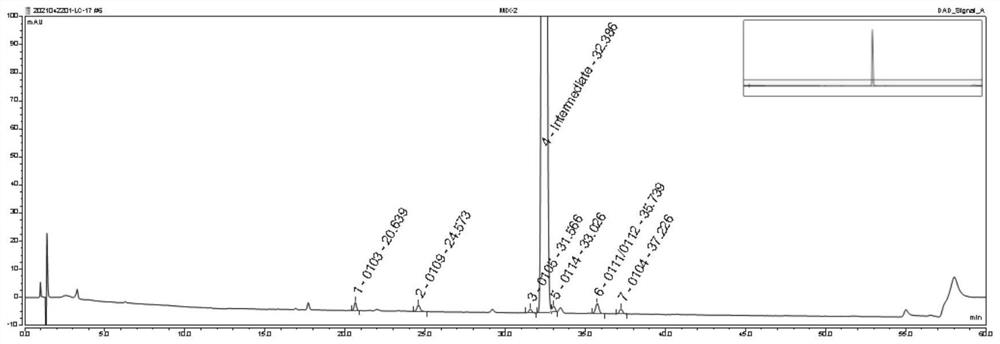
[0069] Preparation of system test solution: Take an appropriate amount of oseltamivir phosphate intermediate and the above impurities 0103, 0104, 0105, 0109, 0111, 0112, 0114, dissolve and dilute with acetonitrile-water (30:70) to obtain oseltamivir phosphate The concentration of the intermediate is 1.0mg / ml, and the system test solution of each impurity is 2μg / ml;
[0070] Preparation of impurity localization solution: Dissolve and dilute appropriate amounts of the above impurities 0103, 0104, 0105, 0109, 0111, 0112, and 0114 with a solvent (acetonitrile-water (30:70)) to obtain a localization solution with a concentration of each impurity of 0.1 mg / ml. solution;
[0071] Use reversed-phase high-performance liquid chromatography to detect, detection conditions:
[0072] Chromatographic column: Chromatographic column model: YMC Pack-Pro C18; Chromatographic column packing: octadecylsilane bonded silica gel; Chromatographic column specifications: inner diameter 3.0mm, length 2...
Embodiment 3
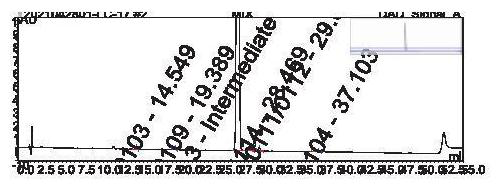
[0082] Preparation of system test solution: Take an appropriate amount of oseltamivir phosphate intermediate and the above impurities 0103, 0104, 0105, 0109, 0111, 0112, 0114, dissolve and dilute with acetonitrile-water (30:70) to obtain oseltamivir phosphate The concentration of the intermediate is 1.0mg / ml, and the system test solution of each impurity is 2μg / ml;
[0083] Preparation of impurity localization solution: Dissolve and dilute appropriate amounts of the above impurities 0103, 0104, 0105, 0109, 0111, 0112, and 0114 with a solvent (acetonitrile-water (30:70)) to obtain a localization solution with a concentration of each impurity of 0.1 mg / ml. solution;
[0084] Use reversed-phase high-performance liquid chromatography to detect, detection conditions:
[0085] Column: Column model: Phenomenex Kinetex PFP Chromatographic column packing: pentafluorophenylsilane bonded silica gel; column specifications: inner diameter 4.6mm, length 150mm, packing particle size 5μm; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com