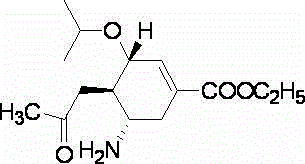
Oseltamivir lyophilized orally-disintegrating tablets and preparation method thereof
A technology for oseltamivir phosphate and orally disintegrating tablets, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of not being easy to take and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Element Content (g) Mannitol 15.000 hydrolyzed gelatin 12.500 Oseltamivir Phosphate 6.569 (equivalent to oseltamivir 5g) Dextran 1.500 aspartame 0.900 milk flavor 0.600
[0040] The preparation steps of freeze-dried orally disintegrating tablet are as follows:
[0041] Dissolution: Add hydrolyzed gelatin (adhesive) to 60ml of water, mix and heat, continue to mix until completely dissolved; add the mixture of mannitol (skeleton agent) and oseltamivir phosphate, stir until uniform, continue to add dextran (lyoprotectant) and aspartame and milk-flavored essence (flavoring agent), stir evenly, dilute the mixture to 100ml with water, stir and mix evenly;
[0042] Injection molding: absorb the liquid medicine and inject the liquid medicine into the mold at 0.2ml / tablet;
[0043] Quick-freezing: The medicine liquid is quickly frozen into a solid under the environment of -60°C;
[0044] Freeze-drying: Cool...
Embodiment 2
[0052] Element Content (g) Mannitol 30.000 Purulan 0.500 Oseltamivir Phosphate 6.569 (equivalent to oseltamivir 5g) Sucralose 1.000
[0053] The preparation steps of freeze-dried orally disintegrating tablet are as follows:
[0054] Dissolution: Add pullulan (adhesive) to 60ml of water, mix and heat to 40°C, continue to mix until completely dissolved; add the well-mixed mixture of mannitol (skeleton agent) and oseltamivir phosphate, and stir until uniform , continue to add triclosan (flavoring agent), stir evenly, dilute the mixed solution to 100ml with water, stir and mix evenly;
[0055] Injection molding: absorb the liquid medicine and inject the liquid medicine into the mold at 0.2ml / tablet;
[0056] Quick-freezing: -120 ℃ environment makes the liquid medicine be frozen into a solid rapidly;
[0057] Freeze-drying: Cool down the freeze-dryer to -20°C, put the mold containing the liquid medicine into the freeze-dryer, v...
Embodiment 3
[0065] Element Content (g) Mannitol 10.000 gelatin 10.000 Oseltamivir Phosphate 6.569 (equivalent to oseltamivir 5g) Dextran 1.200 Sucralose 0.400 sodium bicarbonate 4.600 strawberry flavor 0.200
[0066] The preparation steps of freeze-dried orally disintegrating tablet are as follows:
[0067] Dissolution: Add gelatin to 50ml of water, mix and heat to 40°C, continue to mix until the gelatin is completely dissolved; add a mixture of well-mixed mannitol and sodium bicarbonate and 6.569g of oseltamivir phosphate (equivalent to oseltamivir 5g) , stir until uniform, continue to add dextran, sucralose and strawberry flavor, stir evenly, dilute the mixture to 80ml with water, stir and mix evenly;
[0068] Injection molding: absorb the liquid medicine and inject the liquid medicine into the mold at a rate of 0.4ml / tablet;
[0069] Quick-freezing: the medicine liquid is quickly frozen into a solid under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com