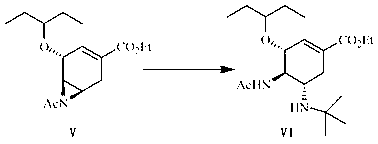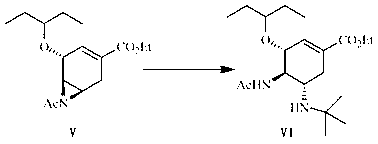Method for synthesizing oseltamivir phosphate without using nitrine
A technology for oseltamivir phosphate and compounds, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve the problem of severe reaction conditions for removing phosphonate groups, not particularly suitable for industrial production, and difficult to achieve. problems such as industrial production routes, to achieve the effects of high yield, convenient operation, and industrial production safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
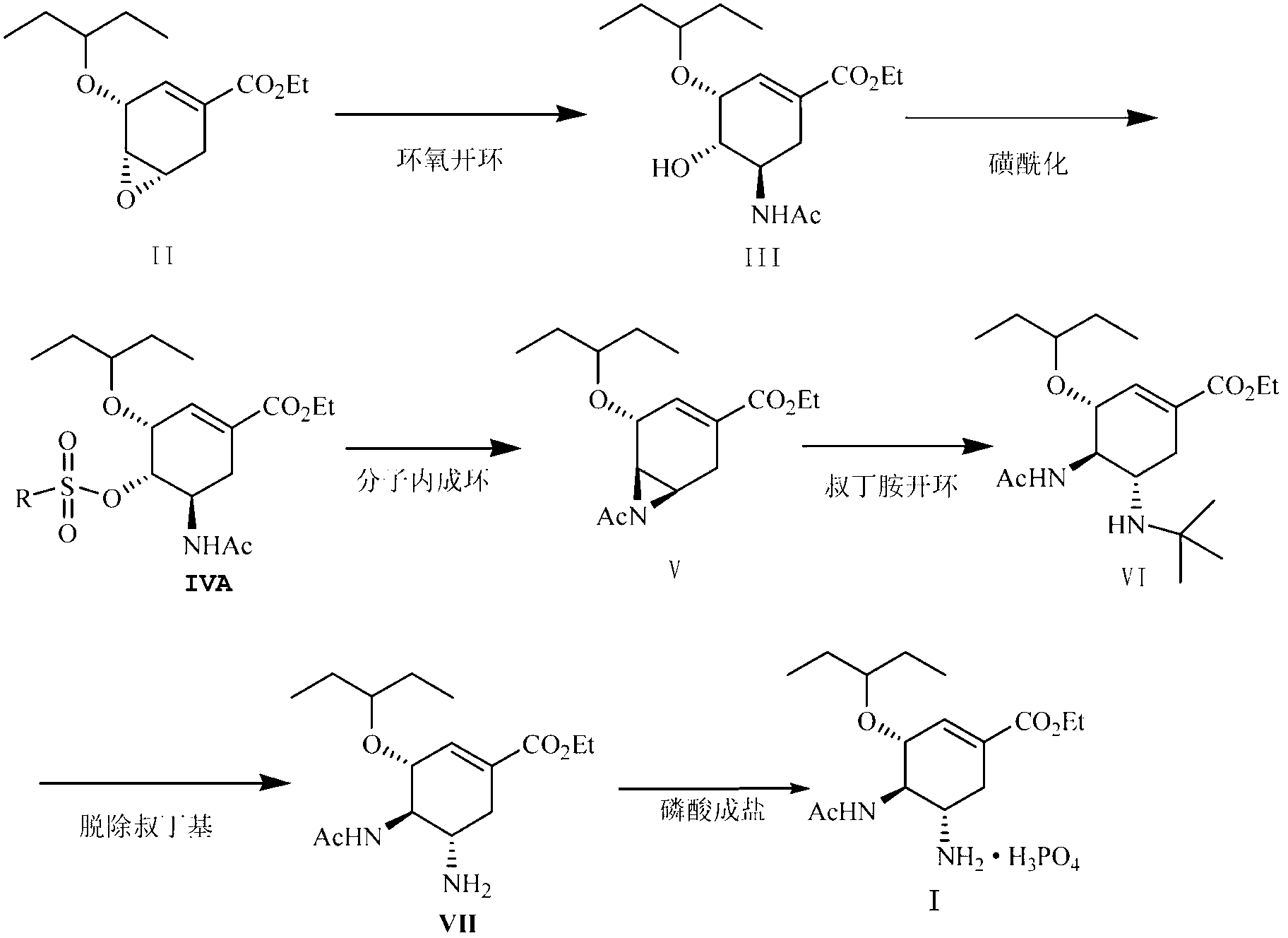
[0065] (3R, 4R, 5R)-4-hydroxy-3-(1-ethylpropoxy)-5-N-acetylamino-1-cyclohexene-1-carboxylic acid ethyl ester (Compound III):
[0066] Ethyl (3R, 4R, 5S)-4,5-epoxy-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate (Compound II) (7.50g, 29.49 mmol ) Place in a flask, add 120 ml acetonitrile, stir at room temperature, slowly add boron trifluoride ether (6.27g, 44.18 mmol), stir at room temperature for 4 h, add 15 ml water, stir at room temperature for 3 h, neutralize with potassium carbonate After stirring at room temperature for 15 hours, adding 150 ml ethyl acetate and 120 ml water for extraction, the resulting organic layer was dried over anhydrous magnesium sulfate, the magnesium sulfate was filtered off, and the filtrate obtained was concentrated to obtain compound III (8.78g, 28.00mmol) with a yield of 95% .
[0067] The performance parameters and spectral data of the obtained compound III are as follows:
[0068] Mp 98.1-98.6 ℃. [α] D 25 = -171.0 ( c 2.0, CHCl 3 )
[0069] 1 H NM...
Embodiment 2
[0072] (3R, 4R, 5R)-4-Methanesulfonyloxy-3-(1-ethylpropoxy)-5-N-acetylamino-1-cyclohexene-1-carboxylic acid ethyl ester (Compound IV ) Preparation:
[0073] Put compound III (6.00 g, 19.14 mmol) in a flask, add 120 ml of ethyl acetate, stir under an ice bath, then add triethylamine (2.90 g, 28.7 mmol) and DMAP (0.23 g, 1.88 mmol), Methanesulfonyl chloride (3.29 g, 28.7 mmol) was slowly dropped into the reaction solution, reacted at 0°C for 0.5 h, 50 ml of water was added and stirred for 0.5 h, the organic layer was separated and washed once with 50 ml of water, and the obtained organic layer was dried with anhydrous magnesium sulfate. The magnesium sulfate was filtered off, and the solvent was evaporated under reduced pressure to obtain compound IV (7.10 g, 18.15 mmol) as a pale yellow oil with a yield of 95%.
[0074] The performance parameters and spectral data of the obtained compound IV are as follows:
[0075] [α] D 25 = -117.7 ( c 1.3, CHCl 3 ).
[0076] 1 H NMR (Acetone-d 6...
Embodiment 3
[0080] Preparation of (3R, 4R, 5R)-4, 5-N-acetylaziridine-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester (compound V):
[0081] Put compound IV (3.00 g, 7.67 mmol) in a flask, then add 90 ml of N,N-dimethylformamide, stir under a water bath, add 60% sodium hydride (0.61 g, 15.31 mmol) in portions, and react 6. h, add 100 ml of dichloromethane, add 40 ml of water under ice bath, extract, and then wash the obtained organic layer with 40 ml of water, and dry the obtained organic layer with anhydrous magnesium sulfate. The magnesium sulfate was filtered off, and the solvent was evaporated under reduced pressure to obtain compound V (2.01 g, 6.81 mmol) as a pale yellow oil with a yield of 89%.
[0082] The performance parameters and spectral data of the obtained compound V are as follows:
[0083] [α] D 20 = –46.0 ( c 1.6, CHCl 3 ).
[0084] 1 H NMR (CDCl 3 ) δ 0.91 (t, J = 7.4 Hz, 3H), 0.96 (t, J = 7.4 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H), 1.48-1.64 (m, 4H), 2.14 (s, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com