Pharmaceutical composition of oseltamivir phosphate coated particles, as well as application and preparation method
A technology of oseltamivir phosphate and oseltamivir phosphate, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, to achieve the effect of stable pharmaceutical composition preparations and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
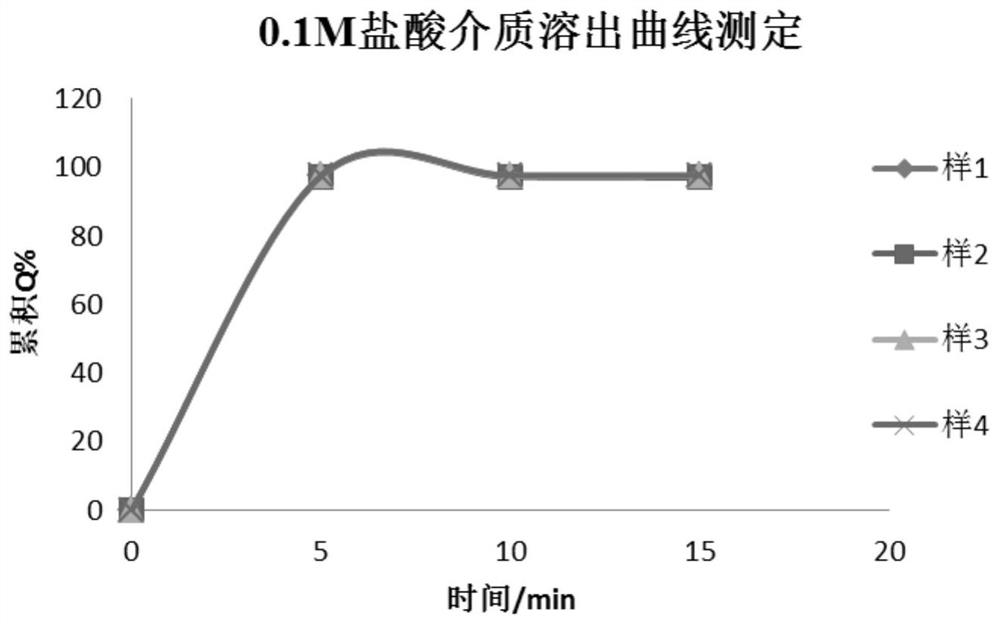
Image
Examples
preparation example Construction
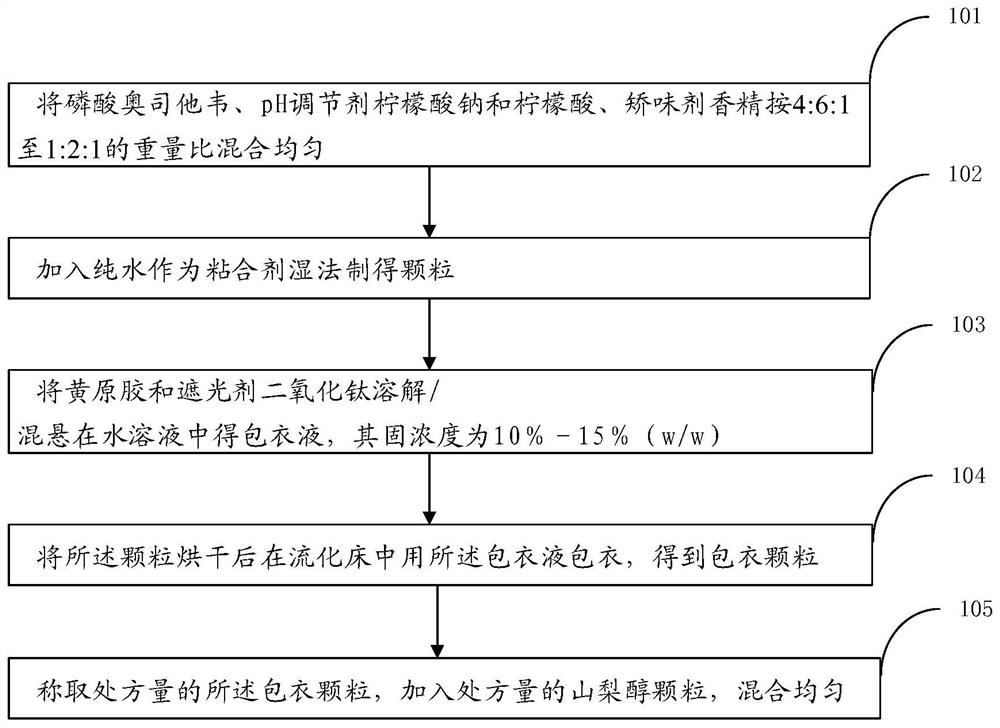
[0035] The present invention also provides a kind of preparation method of dry suspension, described method comprises the following steps:
[0036] (1) Mix oseltamivir phosphate, pH regulator sodium citrate and citric acid, and flavoring agent essence in a weight ratio of 4:6:1 to 1:2:1;
[0037] (2) add pure water to make oseltamivir phosphate drug-containing granule as binder wet method;
[0038] (3) dissolving / suspending xanthan gum and sunscreen agent titanium dioxide in an aqueous solution to obtain a coating solution, the solid concentration of which is 10%-15% (w / w);
[0039] (4) Coating the drug-containing granules with the coating liquid in a fluidized bed after drying to obtain coated granules;
[0040] (5) Weigh the coated granules of the prescribed amount, add the sorbitol granules of the prescribed amount, and mix evenly.
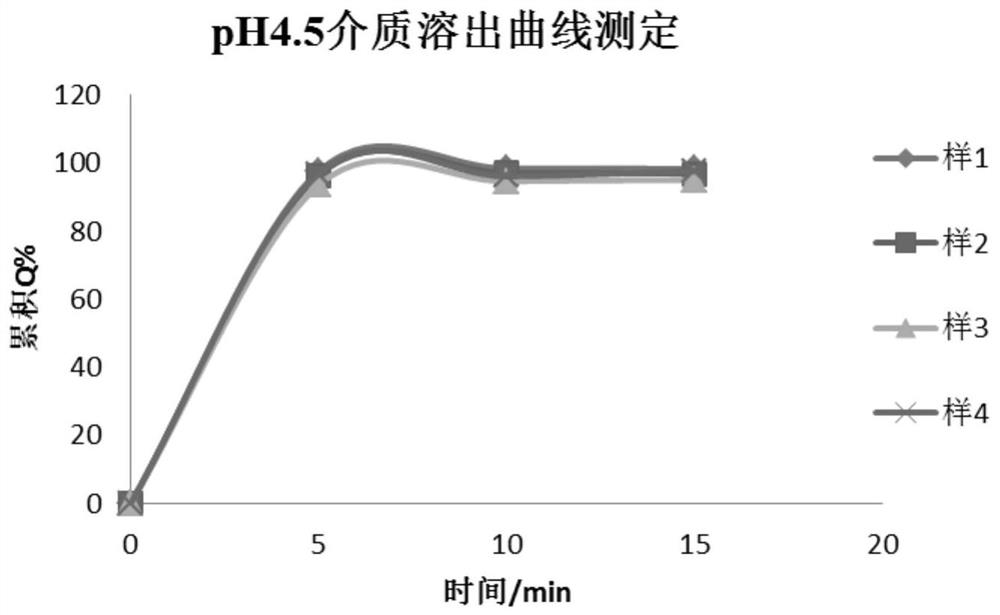
[0041] In the above method, the content of the suspending agent is 60%-80% (w / w). The suspension time of the dry suspension of the present ...
Embodiment 1
[0044] Embodiment 1: Preparation of dry suspension sample 1 containing oseltamivir phosphate
[0045] Preparation prescription for dry suspension:
[0046]
[0047] Preparation Process:
[0048] In addition to the essence, weigh the prescribed amount of oseltamivir phosphate and various auxiliary materials, pass through a 60-mesh sieve, and sorbitol through a 40-mesh sieve, mix uniformly by equal addition method, then add 2.0g of pure water to make a soft material, and pass through a 60-mesh sieve. 30-mesh sieve, dry at 60°C for 1 hour, add essence, pass through 30-mesh sieve for granulation, and mix well.
Embodiment 2
[0049] Embodiment 2: Preparation of dry suspension sample 2 containing oseltamivir phosphate
[0050] Preparation prescription for dry suspension:
[0051]
[0052] Preparation Process:
[0053]All materials are passed through a 40-mesh sieve. Take sodium citrate, citric acid, sorbitol, and xanthan gum and mix evenly, add 2g of pure water as a binder to prepare a soft material, and pass through a 30-mesh sieve to obtain granules. Dry in an oven at 60°C for two hours, then sieve through a 30-mesh sieve, add raw materials, titanium dioxide, and essence to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com