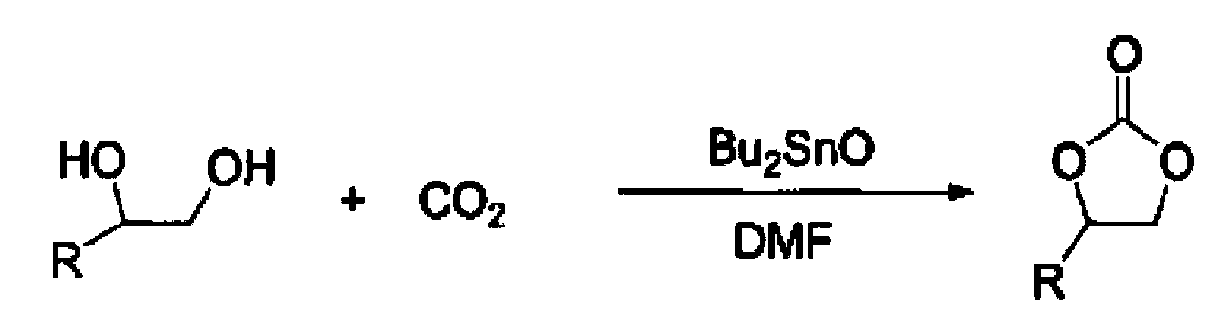Non-isocyan acid water ultraviolet (UV) polyurethane prepared from natural polyhydroxy saccharides and preparation method thereof
A polyhydroxy saccharide and polyurethane technology, which is applied in the field of non-isocyanate water-based UV polyurethane, can solve the problems of worker harm and death danger, and achieve the effect of reducing reaction conditions and raw material requirements, low raw material cost and wide source.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 40 parts of glucose, 0.3 parts of stannous octoate and 5 parts of DMF by weight (all the following examples are the same) to a dry and clean stainless steel autoclave with a stirring volume of 100 ml, heat to 100 ° C, and pass in CO 2 , keep the pressure at 1MPa, stir and react for 3 hours, stop heating and stirring, and slowly vent unreacted CO after cooling 2 , to obtain a cyclic carbonate.
[0033] Take 36 parts of the cyclic carbonate obtained above in a three-necked flask, add 0.05 parts of p-hydroxyanisole and 12 parts of acrylic acid in sequence, and control the temperature at 70°C for 1 hour; after the reaction is complete, cool to room temperature and add 3 parts Tollens reagent, react for 0.5 hours; add 17 parts of triethylamine and stir for 10 minutes, then add 70 parts of diaminodicyclohexylmethane, heat up to 70°C and react for 2 hours, keep warm for 2 hours and then cool to room temperature, that is Get the product.
Embodiment 2
[0035] Add 41 parts of glucose, 0.5 parts of dibutyltin dilaurate and 5 parts of DMF into a dry and clean stainless steel autoclave with a stirring volume of 100 ml, heat to 110 ° C, and pass in CO 2 , keep the pressure at 1.5MPa, stir and react for 3.5 hours, stop heating and stirring, and slowly vent unreacted CO after cooling 2 , to obtain a cyclic carbonate.
[0036] Take 38 parts of the cyclic carbonate obtained above in a three-necked flask, add 0.1 parts of hydroquinone and 14.5 parts of methacrylic acid in sequence, and control the temperature at 80°C for 1.5 hours; after the reaction is complete, cool to room temperature and add 3 parts of Tollens reagent, react for 1 hour; add 19 parts of triethylamine and stir for 10 minutes, then add 55 parts of isophorone diamine, heat up to 75 ° C and react for 2.5 hours, keep warm for 2 hours and cool to room temperature, that is Get the product.
Embodiment 3
[0038] Add 42 parts of glucose, 0.6 parts of dioctyl stannous and 5 parts of DMF into a dry and clean stirred stainless steel autoclave with a volume of 100 ml, heat to 130 ° C, and pass in CO 2 , keep the pressure at 2Mpa, stir and react for 4 hours, stop heating and stirring, and slowly vent unreacted CO after cooling 2 , to obtain a cyclic carbonate.
[0039] Take 40 parts of the cyclic carbonate obtained above in a three-necked flask, add 0.1 parts of p-hydroxyanisole and 15 parts of methacrylic acid in sequence, and control the temperature at 80°C for 2 hours; after the reaction is complete, cool to room temperature, add 5 parts of Fehling reagent, react for 1 hour; then add 20 parts of triethylamine and stir for 10 minutes, then add 66 parts of benzhydrylmethanediamine, heat up to 80°C and react for 3 hours, keep warm for 2 hours and cool to At room temperature, the product was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com