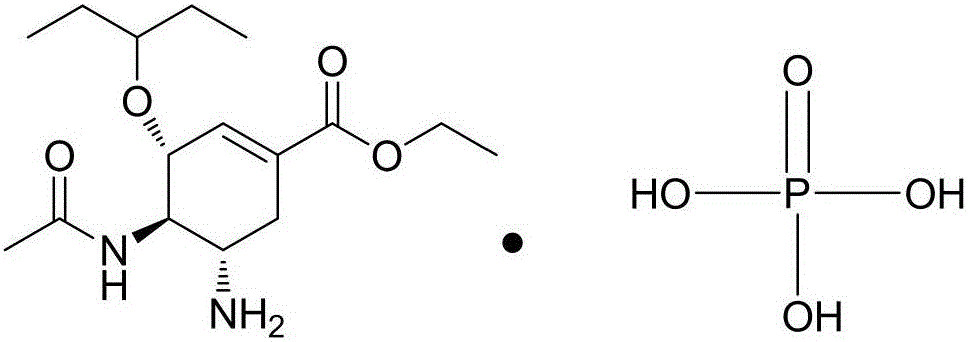
Oseltamivir phosphate tablet and preparation method thereof
A technology of oseltamivir phosphate and oseltamivir phosphate, which is applied in the fields of pill delivery, antiviral agents, and pharmaceutical formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0069] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0070] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
Embodiment 1
[0071] Example 1 Effect of different sweeteners on the stability and mouthfeel of oseltamivir phosphate orally disintegrating tablets
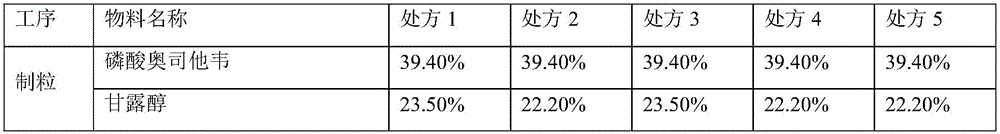
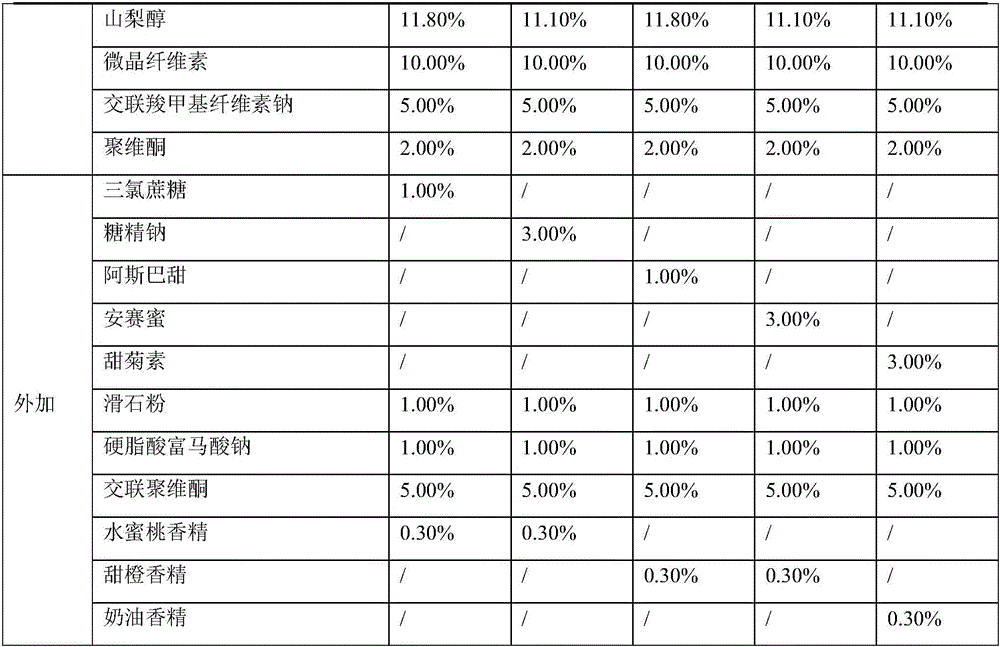
[0072] Prescription
[0073]
[0074]
[0075] Preparation:
[0076] Add oseltamivir phosphate, mannitol, sorbitol, microcrystalline cellulose, croscarmellose sodium and povidone into the granulation pot and pre-mix evenly in the prescription amount, and the blade speed of the granulator is 150rpm. The cutter speed is 1500rpm. A peristaltic pump is used to add 17% to 23% by weight of purified water to the premix for granulation. The peristaltic pump speed is 100rpm, the nozzle aperture is 1.0mm, the blade speed of the granulator during the liquid addition and granulation process is 150rpm, and the cutter speed is 3000rpm. Fluidized bed drying is adopted, and the inlet air temperature is 55°C; the dried granules are granulated through a 045R sieve with a comil granulator. In dry granules, add sucralose or sodium saccharin or aspartame ...
Embodiment 2
[0103] Example 2 Research on prescriptions of different specifications of oseltamivir phosphate orally disintegrating tablets
[0104] Prescription
[0105]
[0106] Preparation method with reference to the preparation method of embodiment 1
[0107] The above-mentioned gained oseltamivir phosphate orally disintegrating tablet is carried out mouthfeel investigation, and investigation result and method are as follows:
[0108] Recruit 20 volunteers, take an orally disintegrating tablet orally, without chewing, hold it in the mouth for 30 seconds, and then spit it out. The interval between individual trials is greater than or equal to 1 day; then let the volunteers answer the questions in the questionnaire; evaluate the key issues.
[0109]
[0110]
[0111] The disintegration time and friability of the oseltamivir phosphate orally disintegrating tablets prepared by prescriptions 1 and 6-9 described in Example 2 were carried out.
[0112] Disintegration time and friab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com