Preparation method of oseltamivir phosphate capsule
A technology of oseltamivir phosphate and capsules, which is applied in the field of preparation of oseltamivir phosphate capsules, can solve problems such as poor dissolution uniformity, and achieve the effects of ensuring uniformity and ensuring dissolution uniformity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
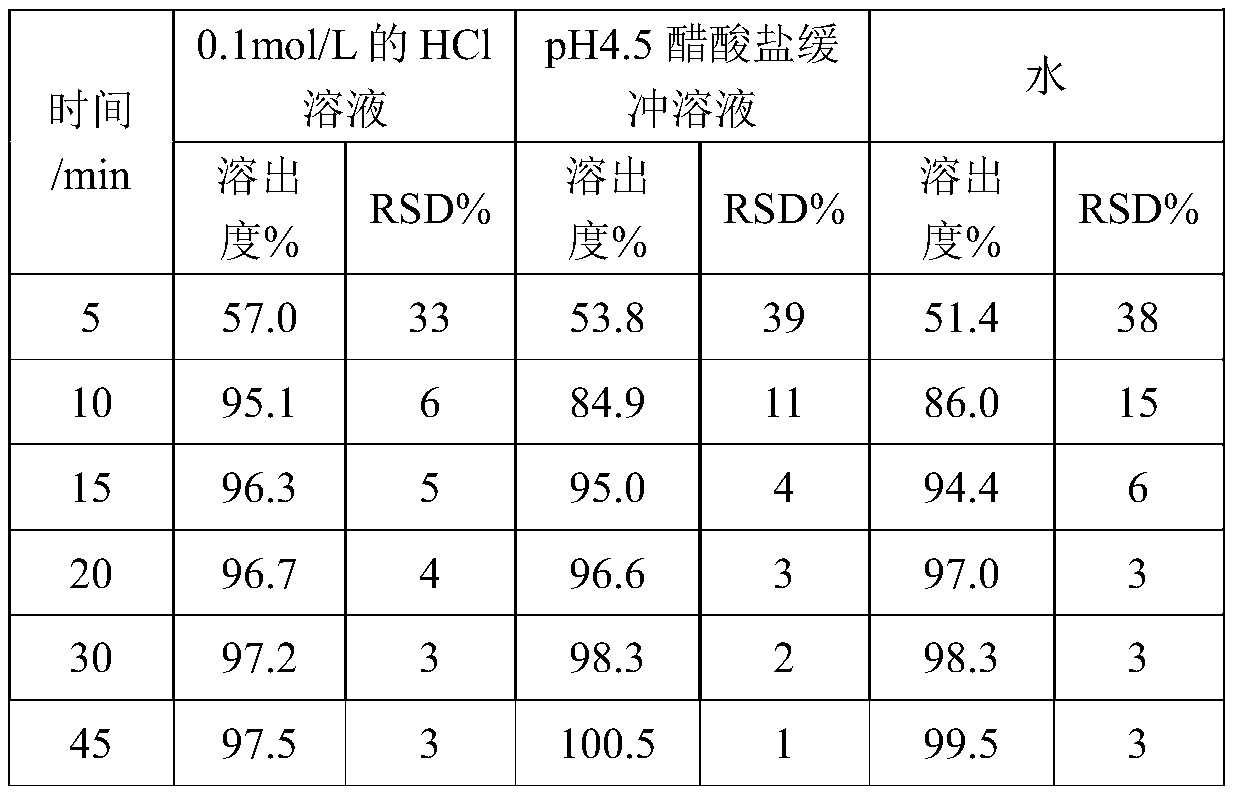
[0050] Embodiment 1: The influence of same prescription, different preparation methods on dissolution uniformity
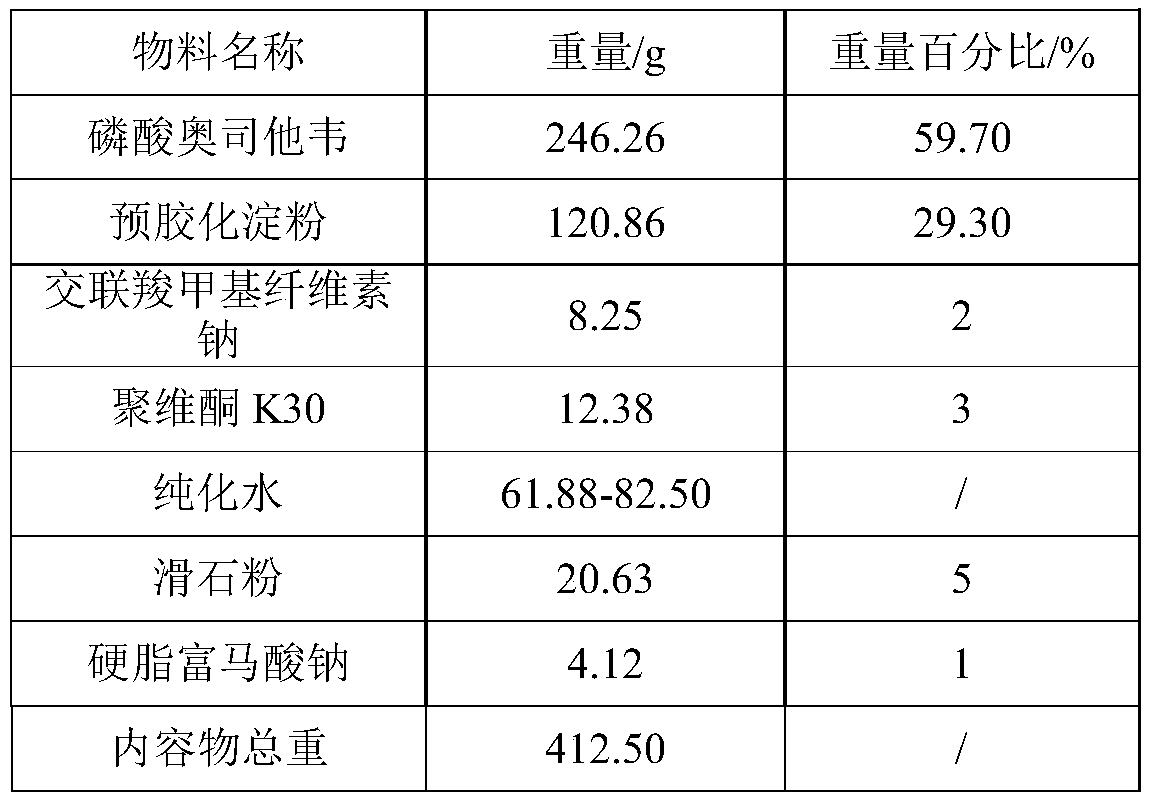
[0051] 1. Prescription information
[0052]
[0053] 2. Different preparation methods
[0054] Adopt different preparation methods to prepare respectively the capsules of 2500 grain amounts, concrete method is as follows:
[0055] Method A:
[0056] (1) Weigh the prescription amount of oseltamivir phosphate, pregelatinized starch, povidone K30, croscarmellose sodium and prescription amount of talcum powder and place them in the wet mixing granulator, mix at 100rpm 5min.
[0057] (2) Weigh an appropriate amount of purified water as a wetting agent.
[0058] (3) Add the wetting agent described in step (2) into the wet mixing granulator, mix at 150rpm for 2min, and granulate.
[0059] (4) After drying the granules obtained in step (3), add the prescribed amount of sodium stearyl fumarate, and mix for 5 minutes to obtain a mixture.
[0060] (5) Fill capsules ...
Embodiment 2
[0080] Embodiment 2: The impact of the proportion of glidants added in batches on the uniformity of dissolution
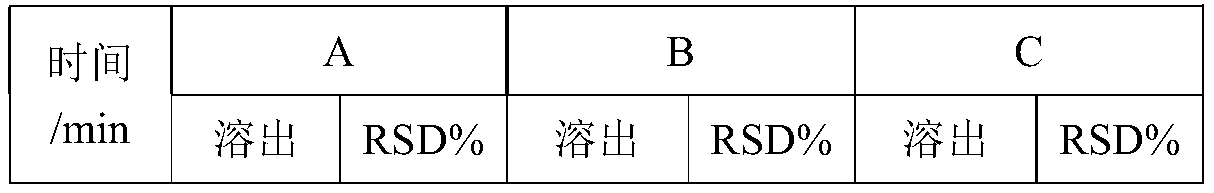
[0081] Adopt the prescription of Example 1 and the preparation method of method C, only change the addition ratio of the glidant twice (see Table 3 for the specific ratio, the percentage of the prescription), and other conditions are exactly the same, and prepare 2500 capsules. Each group took 12 capsules respectively, and adopted the same method as in Example 1 to detect the dissolution rate, and the results are shown in Table 4.
[0082] Table 3 Different proportions of glidants
[0083] Example Glidant for mixing in step (1) Glidant for mixing in step (4) 2-1 30% 70% 2-2 40% 60% 2-3 50% 50% 2-4 60% 40% 2-5 70% 30%
[0084] Table 4 Dissolution Data
[0085]
[0086]
[0087] As can be seen from Table 3 and Table 4, first mix 30%-70% glidant with bulk drug, filler, binder, and disintegrant, then add water to g...
Embodiment 3
[0088] Embodiment 3: the influence of different excipients on dissolution uniformity
[0089] According to the following prescription, the preparation process of Example 2-3 was used to prepare 2500 tablets. And the method in Example 1 was adopted to detect the content uniformity and dissolution rate of the following prescriptions, and the results are shown in Table 5.
[0090] prescribing information
[0091]
[0092] Table 5 Dissolution Data
[0093]
[0094]
[0095] As can be seen from Table 5, changing the type of adjuvant in the prescription, using the method C of the present invention can also control the RSD of the dissolution rate at each detection time point within 10%, and the dissolution uniformity of the preparation is better. It is suggested that the way of adding glidant is the key factor to improve the dissolution uniformity of oseltamivir phosphate capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com