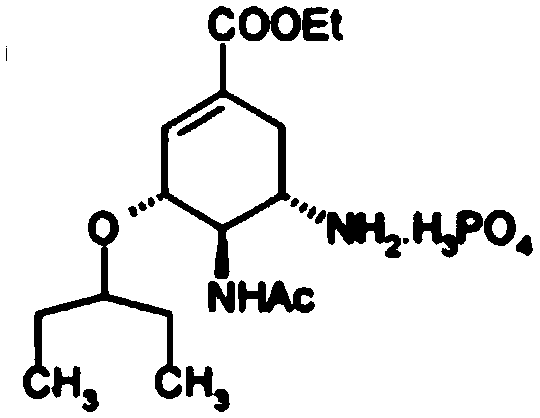
Oseltamivir phosphate preparation method
A technology of oseltamivir phosphate and trifluoroacetic acid, which is applied in the field of preparation of oseltamivir phosphate, can solve the problems that the heavy metals in the product are not easy to meet the standard, the recovery of precious catalysts is difficult, and the reuse cannot be performed, and the preparation method is simple and heavy. The effect of good performance, improved quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
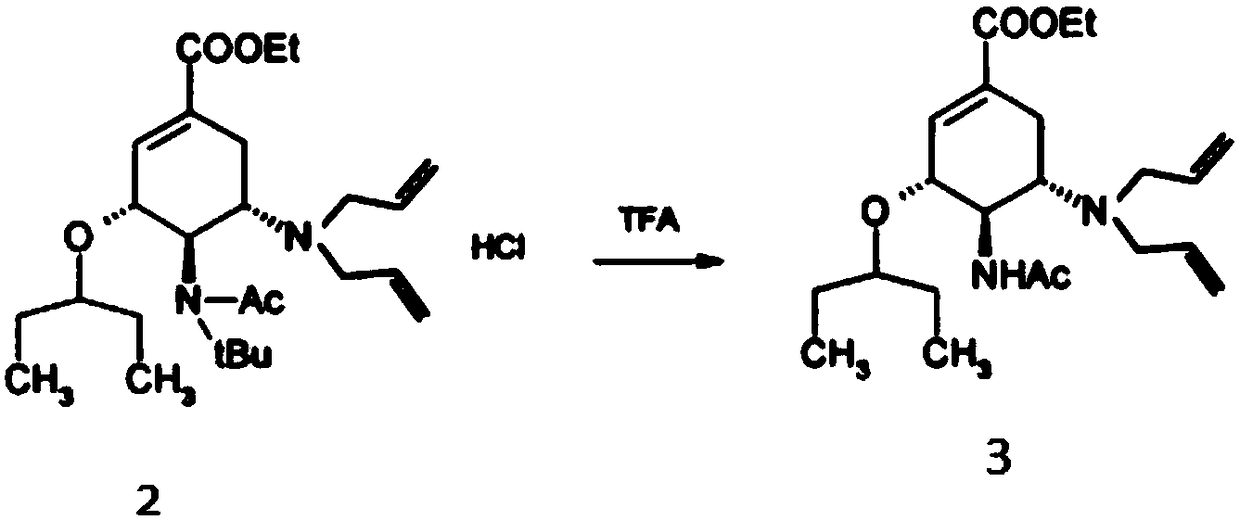
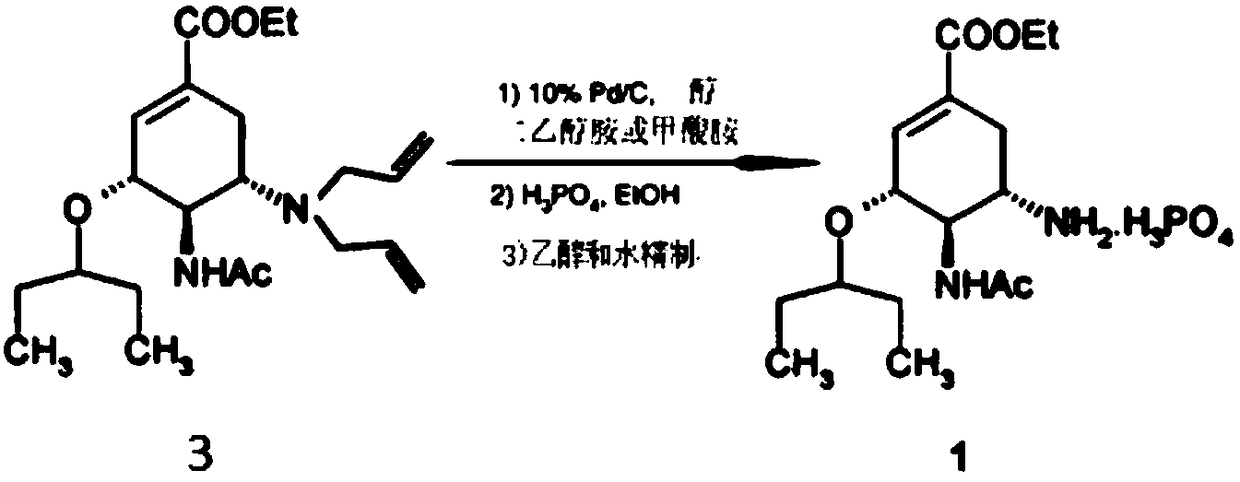
[0024] 1. Add 541.5g (1.116mol, HPLC99.989%) of compound 2 into 700mL of trifluoroacetic acid, stir, heat up to 45-55°C, and keep it warm for 3 hours; cool the reaction solution to 20-30°C, add 250mL of toluene , the reaction solution was concentrated under reduced pressure in a hot water bath at 45-55°C until no liquid flowed out. Add 1000mL of toluene to the concentrate, cool down to 0-10°C, and add 300mL of cold water to maintain 0-10°C; control the temperature at 0-10°C, slowly Add 158.75g (3.969mol) of sodium hydroxide solution in water (500mL) dropwise, adjust the pH to about 12-13, let it stand for stratification, extract the water layer with 500mL of toluene, let it stand for separation, combine the organic layers and wash each time with 250mL of water Three times, stand still and separate layers; the organic layer is dried with 50g of anhydrous sodium sulfate, filtered, the combined filtrate is concentrated under reduced pressure in a hot water bath at 45-55°C until no...
Embodiment 2
[0027] In this example, the diethanolamine in Example 1 was replaced with equimolar ammonium formate, and the other steps were the same as in Example 1 to obtain 85.7 g of white solid oseltamivir phosphate crude product, with an HPLC purity of 99.854% and a single largest impurity of 0.083%; Add 85.7g of crude oseltamivir phosphate to 3428mL of ethanol aqueous solution with a volume concentration of 99.2%, raise the temperature to reflux to dissolve, lower the temperature to 65-70°C, add 4.3g of activated carbon, raise the temperature to reflux, stir for 30 minutes, filter, and the filtrate Cool down to 45-55°C, there is solid precipitation, continue to stir and cool down to below -10°C, stir for 2 hours, filter, and vacuum-dry the filter cake at 50-60°C to obtain 84.3g of oseltamivir phosphate as a white solid crystal form A , yield 94.85%, HPLC purity 99.913%, single largest impurity 0.054%.
Embodiment 3
[0029]The palladium charcoal recovered in step 2 of the above example 1 was re-reacted according to the method of step 2, and 86.5g of white solid oseltamivir phosphate crude product could be obtained, with an HPLC purity of 99.854% and a single largest impurity of 0.083%; 86.5g of oseltamivir phosphate Add the crude Tasvir to 3460mL ethanol aqueous solution with a volume concentration of 99.2%, heat up to reflux to dissolve, cool to 65-70°C, add 4.3g of activated carbon, heat up to reflux, stir for 30 minutes, filter, and cool the filtrate to 45-55°C , there is solid precipitation, continue to stir and cool down to below -10°C, stir for 2 hours, filter, and vacuum-dry the filter cake at 50-60°C to obtain 84.8g of oseltamivir phosphate in crystal form A, with a yield of 95.41%, HPLC purity 99.908%, single largest impurity 0.055%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com