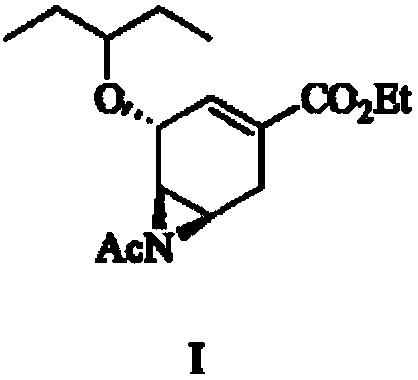
Preparation method of oseltamivir acetyl aziridine intermediate
A technology of viracetaziridine and intermediates, applied in the field of pharmaceutical chemical industry, can solve problems such as low yield, potential safety hazard, harsh reaction conditions, etc., and achieve the effects of abundant raw material sources, increased production capacity, and easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
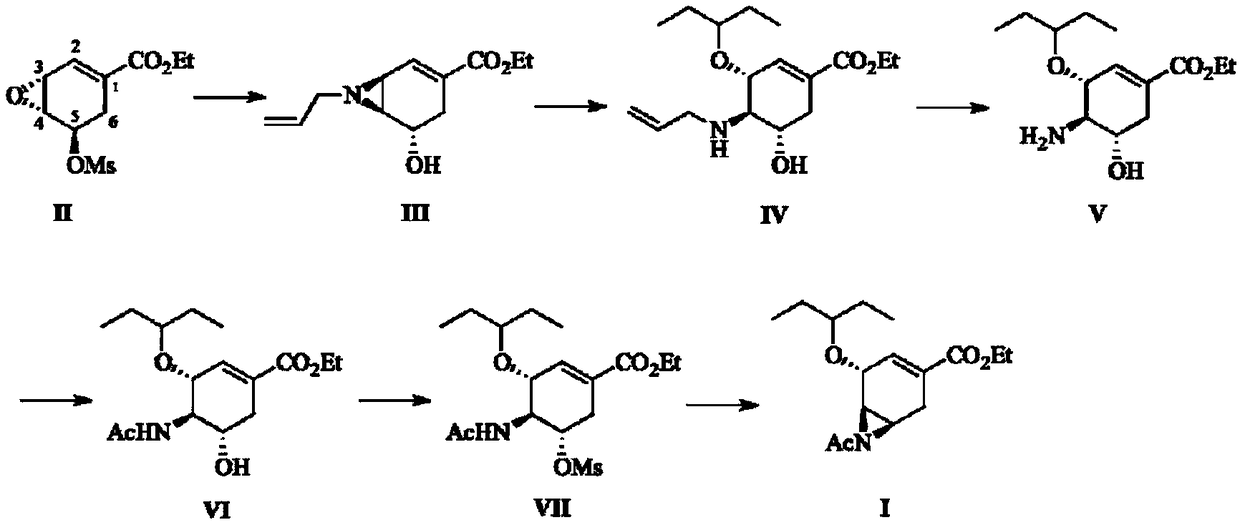
[0024] Preparation of ethyl 3,4-allylaziridine-5-hydroxyshikimate, compound Ⅲ:
[0025] Get 66mL of water and 33mL of absolute ethanol and join in the 250mL one-necked flask, add allylamine (3.226g, 57.3mmol) wherein, then the 3,4-allyl aziridine-5 of gained in embodiment 4 -Ethyl hydroxyshikimate, that is, compound II (5.003g, 19.1mmol), was added to it, heated to 75°C and stirred, reacted for 5 hours, cooled down to room temperature, added 100mL ethyl acetate for extraction, and the aqueous layer was washed with 50mL acetic acid Ethyl was washed twice, the organic layers were combined and dried with anhydrous magnesium sulfate, the solid magnesium sulfate was filtered off, ethyl acetate and part of ethanol were distilled off under reduced pressure to obtain a light yellow liquid, compound III (3.947 g, 17.7 mmol), yield 93%. Purified by column chromatography for structure identification.
[0026] Compound III was detected by nuclear magnetic resonance apparatus, and the re...
Embodiment 2
[0029] 3-(1-ethylpropoxy)-4-allylamino-5-hydroxyethyl oxalate is the preparation of compound IV:
[0030] Compound III (6.004g, 26.9mmol) obtained in Example 1 was placed in a 250mL single-necked flask, and 30mL of 3-pentanol was first added to dissolve it, and boron trifluoride diethyl ether (5.735g, 40.4mmol) was added thereto at room temperature, and heated to React at 70°C for 3 hours. After TLC monitors that the raw materials disappear completely, cool the reaction solution to room temperature, add 100mL ethyl acetate and water to it, stir, and add potassium carbonate solid to adjust the pH to about 10, separate the liquid, and use ethyl acetate The aqueous layer was washed twice (2×50 mL), the ethyl acetate was combined and dried, and the ethyl acetate and 3-pentanol were evaporated to obtain compound IV (7.526 g, 24.2 mmol) as a yellow liquid with a yield of 90%. Purified by column chromatography for structure identification.
[0031] Compound Ⅳ was detected with a nuc...
Embodiment 3
[0034] 3-(1-Ethylpropoxy)-4-amino-5-hydroxyshikimate ethyl ester is the preparation of compound V:
[0035] Compound IV (5.003 g, 16.1 mmol) obtained in Example 2 was placed in a 250 mL one-necked flask, dissolved in 100 mL of ethanol, and 1,3-dimethylbarbituric acid (2.761 g, 17.7 mmol) was added at room temperature, Then palladium acetate (36mg, 0.16mmol) and triphenylphosphine (168mg, 0.64mmol) were added, protected with nitrogen, and heated to 35°C for 2 hours to complete the reaction. Then distill off ethanol at about 30°C, add 100mL ethyl acetate and 5%wt potassium carbonate aqueous solution to each of them, extract and separate the layers and wash the aqueous layer twice (2×50mL) with ethyl acetate, combine the ethyl acetate with anhydrous After drying over magnesium sulfate, the ethyl acetate was distilled off to obtain a yellow liquid, compound V (4.065 g, 15.0 mmol), with a yield of 93%. Purified by column chromatography for structure identification.
[0036] Compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com