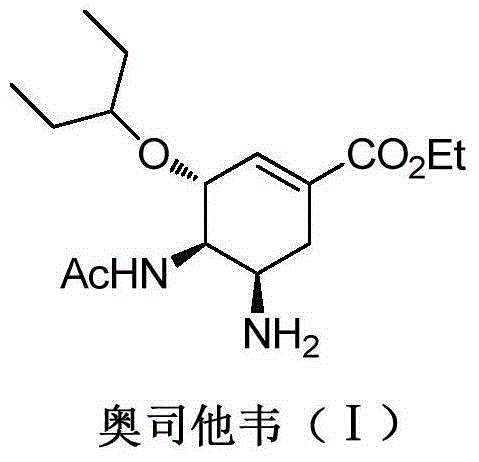
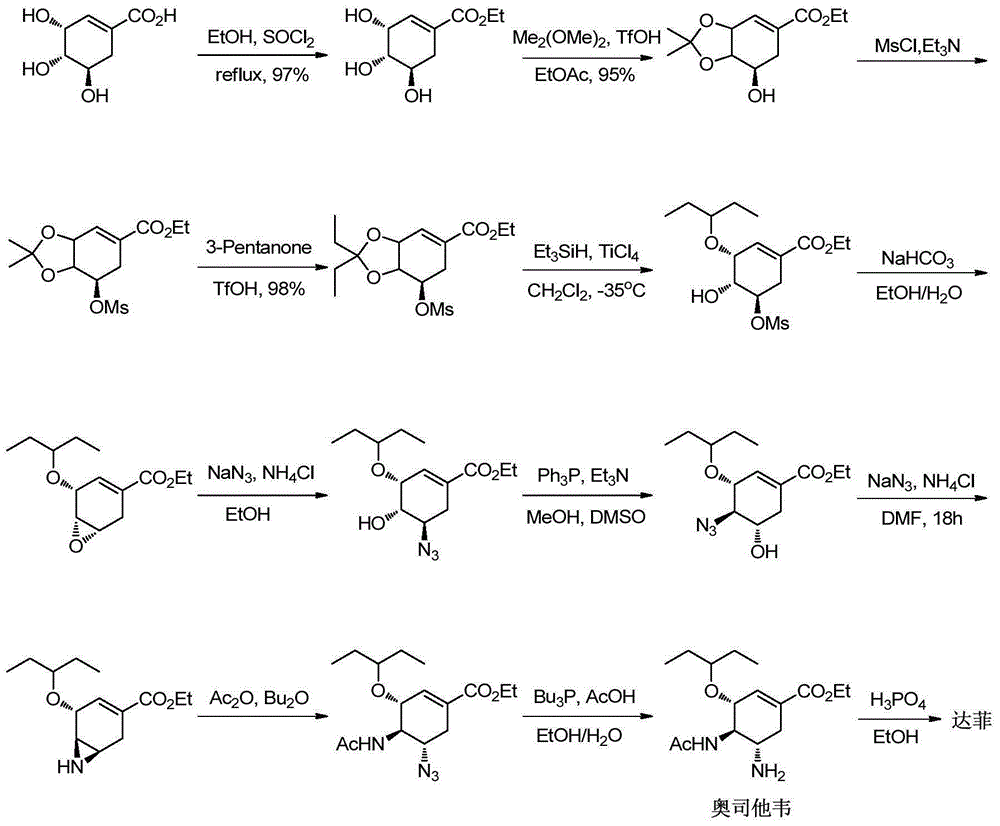
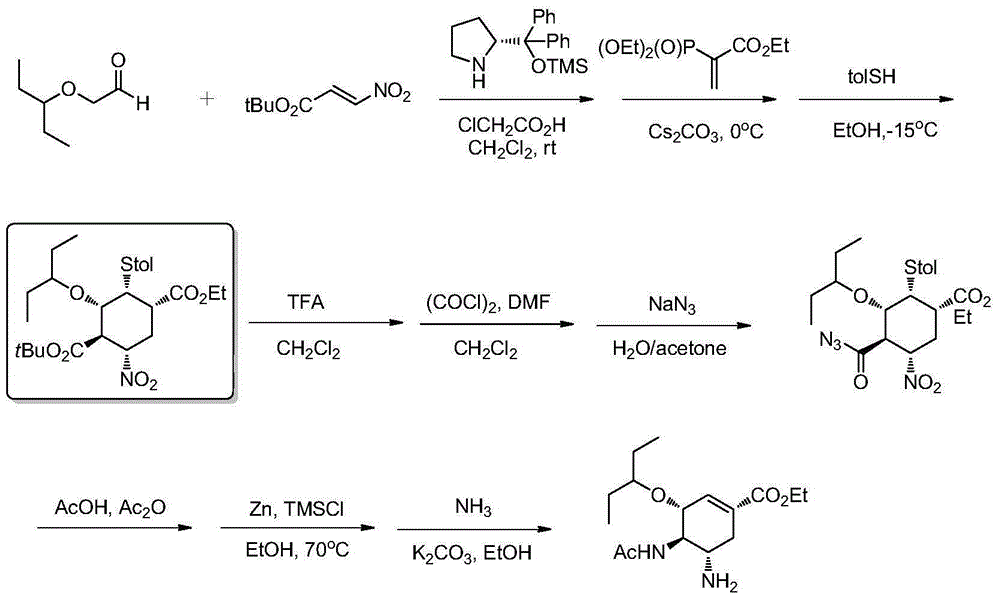
New preparation method of oseltamivir intermediate
A technology of oseltamivir and intermediates, applied in the field of compound synthesis, can solve the problems of inability to significantly improve the total yield, unsafe, limited sources, etc., achieve great feasibility and prospects, cheap and easily available reagents, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of compound shown in formula III
[0054]
[0055] Add the compound shown in formula II (510.0mg, 1.0mmol) and 10mL of methanol to a 50mL reaction bottle to dissolve, add 2mL of 2mol / L hydrochloric acid, stir at room temperature, and react for about 6 hours. TLC traces the reaction to the compound shown in structural formula II. After the disappearance of the starting compound, the reaction was stopped, and the solvent was removed by rotary evaporation to obtain a substantially pure compound represented by structural formula III with a yield of 98%.
Embodiment 2
[0057] The preparation of compound shown in formula IV
[0058]
[0059]The compound shown in formula III (453mg, 1.0mmol) was dissolved in 20mL of dichloromethane, cooled to 0°C, and ethyl chloroformate (109mg, 1.0mmol) and triethylamine (202mg, 2.5mmol) were added to react for half an hour and gradually React at room temperature for 1 hour, remove the solvent by rotary evaporation, add hydroxylamine hydrochloride (104 mg, 1.5 mmol) and 20 mL of methanol, stir at room temperature, stop the reaction after about 4 hours of reaction, remove the solvent by rotary evaporation, add about 30 mL of water to the system, Separate the organic phase, add 5 mL of 1N dilute hydrochloric acid, extract with dichloromethane (20 mL × 3), combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent by rotary evaporation to obtain the substantially pure compound shown in formula IV. The yield 92%.
[0060] 1HNMR (400MHz, CDCl3) 8.02(s, 1H), 7.38(d, J=8.0Hz, 2H), 7.07...
Embodiment 3
[0063] The preparation of compound shown in formula V
[0064]
[0065] Dissolve the compound shown in Formula IV (454mg, 1.0mmol) in 10mL of dichloromethane, then add triethylamine (166μL, 1.2mmol), 4mL of acetic anhydride, react at room temperature for about half an hour, stop the reaction, and rotavap to obtain essentially The pure compound represented by formula V has a yield of 99%.
[0066] 1HNMR (400MHz, CDCl3) 7.38 (d, J = 8.0Hz, 2H), 7.07 (d, J = 8.0Hz, 2H), 4.70-4.80 (m, 1H), 4.05-4.17 (m, 1H), 4.02 ( t,J=3.2Hz,1H),3.86-3.98(m,1H),3.75(m,1H),3.54(m,1H),3.17(quintet,J=4.8Hz,1H),2.76(dt,J =13.2,3.6Hz,1H),2.68(dt,J=13.2,3.6Hz,1H),2.31(s,3H),2.28(q,J=13.2Hz,1H),2.17(s,3H),1.28 -1.48(m,2H),1.23(t,J=7.2Hz,3H),1.03-1.18(m,2H),0.76(t,J=7.2Hz,3H),0.63(t,J=7.2Hz, 3H);
[0067] HRMS (ESI) 533.1937 [(M+Na)+].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com