Orally disintegrating tablet suitable for infants and children and preparation method thereof
A technology for oral disintegrating tablets, infants and young children, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve the problems of drug pollution, low hardness packaging and transportation, and drug taste problems. , to achieve the effect of reducing pollution and waste, ensuring divided doses, and no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
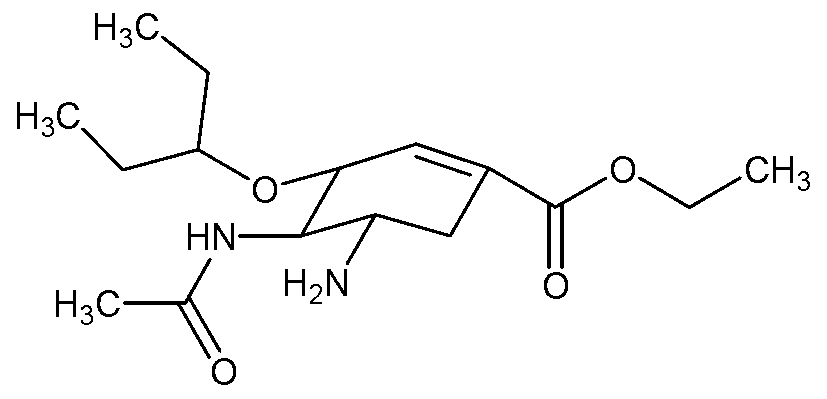
[0094] Embodiment 1 Oseltamivir orally disintegrating tablet and preparation method thereof
[0095] Oseltamivir taste-masked pellets are firstly prepared, and then formulated and compressed according to the prescription to obtain tablets, namely oseltamivir orally disintegrating tablets.
[0096] (1) Preparation process of oseltamivir taste-masked pellets
[0097] All the required raw and auxiliary materials were crushed through a 100-mesh sieve, 150.0g of starch and 150.0g of oseltamivir were put into the centrifugal coating pot, the temperature of the centrifugal coating pot was adjusted to 45°C, and the air intake was adjusted to 65m 3 *h -1 , Add 300.0g of 75% (mass ratio) ethanol to the centrifugal coating pot with a peristaltic pump at a flow rate of 3ml / min. The atomization pressure is 1.0bar. Gradually increase the liquid supply rate to 6ml / min until the adhesive After the solution is sprayed and the coating is completed, continue to dry in a centrifugal coating pan...
Embodiment 2
[0105] Embodiment 2 Oseltamivir orally disintegrating tablet and preparation method thereof
[0106] Basically the same as Example 1, the difference is:
[0107] Orally disintegrating tablets: Oseltamivir taste-masking pellets 107.5g, mannitol 176.4g, microcrystalline cellulose 25.2g, povidone 8.4g, crospovidone 33.6g, aspartame 8.4g , citric acid 2.5g, strawberry essence 2.5g, sodium stearate fumarate 5.0g.
[0108] After calculation, the weight percentage of each component in the oseltamivir orally disintegrating tablet in this example is:
[0109] Oseltamivir taste-masked pellets 29.1%, filler mannitol 47.7%, filler microcrystalline cellulose 6.8%, binder povidone 2.3%, disintegrant crospovidone 9.1%, flavoring agent Aspartame 2.3%, flavoring agent citric acid 0.7%, flavoring agent strawberry essence 0.7%, lubricant sodium stearate fumarate 1.4%.
Embodiment 3
[0110] Embodiment 3 Oseltamivir orally disintegrating tablet and preparation method thereof
[0111] Basically the same as Example 1, the difference is:
[0112] Orally disintegrating tablets: Oseltamivir taste-masked pellets 118.4g, mannitol 350.0g, microcrystalline cellulose 70.0g, povidone 16.8g, low-substituted hydroxypropyl cellulose 47.7g, aspartame 17.9g, citric acid 5.6g, magnesium stearate 8.3g.
[0113] After calculation, the weight percentage of each component in the oseltamivir orally disintegrating tablet in this example is:
[0114] Oseltamivir phosphate taste-masked pellets 18.7%, filler mannitol 55.1%, filler microcrystalline cellulose 11.0%, binder povidone 2.6%, disintegrant low-substituted hydroxypropyl cellulose 7.5%, Flavoring agent aspartame 2.8%, flavoring agent citric acid 0.9%, lubricant magnesium stearate 1.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com