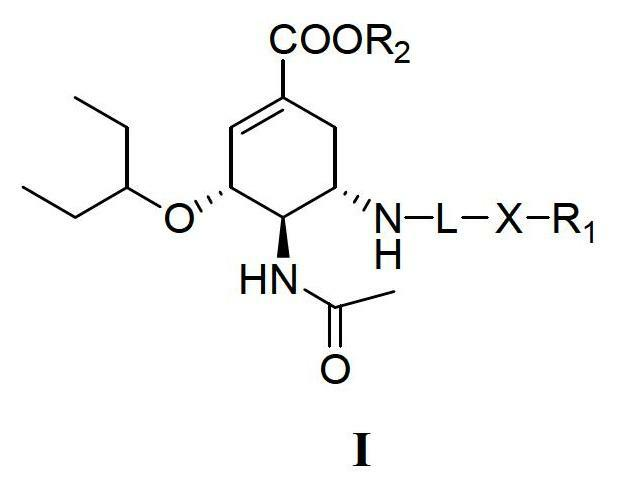
Derivatives of oseltamivir, and method and medical application thereof
A pharmaceutical and drug technology, applied in the field of oseltamivir derivatives and infectious disease drugs caused by influenza virus, can solve problems such as side effects, virus drug resistance central nervous system, and clinical application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
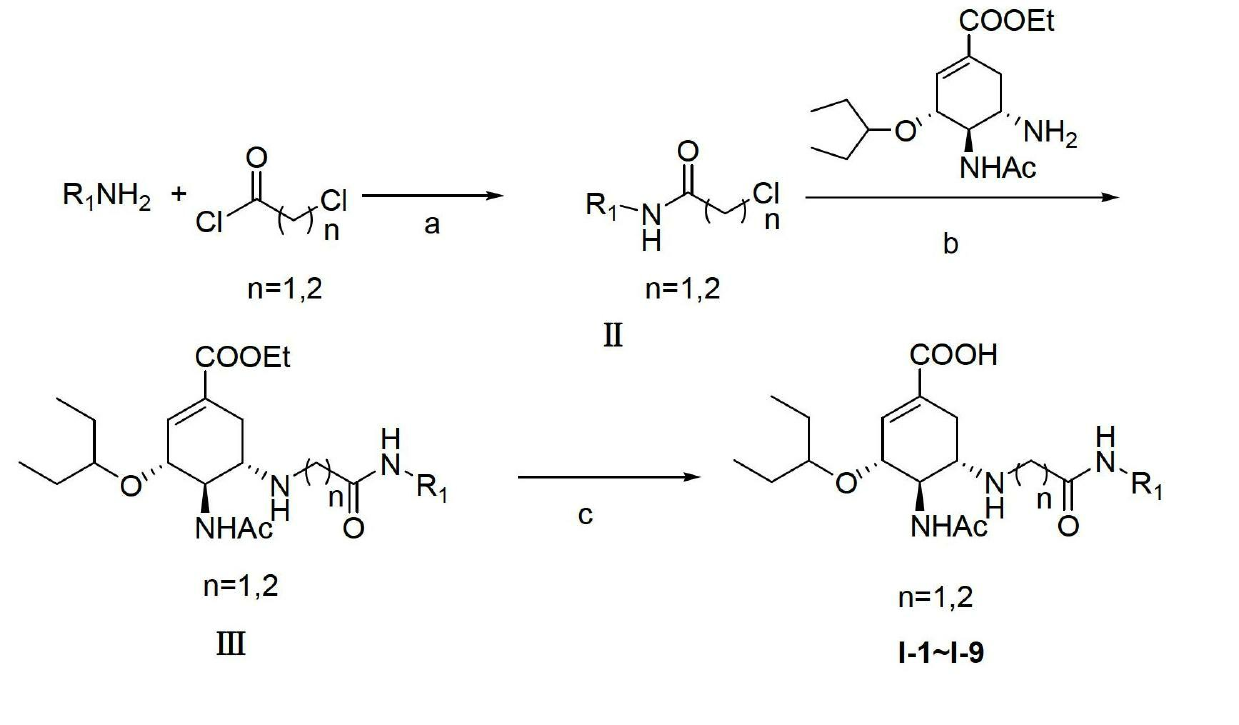
[0091] 2-Chloro-N-(4-chloro-2-methylphenyl)acetamide (II)
[0092] Add CH to o-methyl-p-chloroaniline (1.42g, 10mmol) 2 Cl 2 Dissolve, add triethylamine 2ml, dropwise add the CH of chloroacetyl chloride (0.93g, 12mmol) in system 2 Cl 2 Solution, the system produces a lot of white smoke, and the purple clear solution turns dark green. After the dropwise addition was completed, the ice bath was removed, and the mixture was stirred at room temperature for 1 hour. Add 20ml CH 2 Cl 2 Diluted, saturated NH 4 Wash twice with Cl, CH 2 Cl 2 Anhydrous Na 2 SO 4 Dry for 1 h, spin dry CH 2 Cl 2 , added PE to wash, and suction filtered to obtain 1.41 g of a gray product, with a yield of 64.68%. m.p.132-133℃
Embodiment 2
[0094] (3R, 4R, 5S)-4-acetylamino-5-(2-(4-chloro-2-methylanilino)-2-oxoethylamino)-3-(1-ethylpropoxy )-1-cyclohexene-1-carboxylic acid ethyl ester (III)
[0095] Add oseltamivir 3.1g (10mmol), 2-chloro-N-(4-chloro-2-methylphenyl)acetamide 2.4g (12mmol), KI0.17 (1mmol) DMF in 100ml single-necked bottle 20mL, react at 45-55℃ for about 14h. After the reaction was detected by TLC, the reaction solution was poured into water, and the system precipitated a fine powdery solid, extracted 3 times with 70 mL of ethyl acetate, and the organic layer was combined, and anhydrous NaSO 4 dry. Silica gel column chromatography (ethyl acetate:methanol=10:1) yielded 1.68 g of a brown oily product as a solid, with a yield of 34%.
[0096] IR(KBr)v max (cm -1 ) 3588, 3568, 3448, 2360, 2342, 1685, 1654, 1647, 1637, 1629, 1618, 1384, 669;
[0097] 1 H NMR (CDCl 3 )0.86-0.91 (6H, m), 1.27-1.32 (4H, t, J=15.0Hz), 1.43-1.62 (4H, m), 1.93 (3H, s), 2.18 (3H, s), 2.17 (3H , s), 2.27(2H, s), 2.41-2...
Embodiment 3
[0100] (3R, 4R, 5S)-4-acetylamino-5-(2-(4-chloro-2-methylanilino)-2-oxoethylamino)-3-(1-ethylpropoxy )-1-cyclohexene-1-carboxylic acid (I-1)
[0101] Add (3R, 4R, 5S)-4-acetylamino-5-(2-(4-chloro-2-methylanilino)-2-oxoethylamino)-3-(1 -Ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester 1.4g (2.8mmol), then add 20mLTHF to dissolve, add 1mol / L LiOH15mL, stir at room temperature for 4h. After the reaction was detected by TLC, THF was removed by rotary evaporation. Concentrated hydrochloric acid was added dropwise under ice bath conditions to adjust the pH to 3-4. A khaki solid precipitated out of the system. Suction filtration and drying of the filter cake yielded 1.12 g of the product, with a yield of 85%. m.p.186-190°C.
[0102] IR(KBr)v max (cm -1 )3650, 3415, 2965, 2360, 2342, 1685, 1654, 1647, 1637, 1545, 1484, 1384, 669;
[0103] 1 H NMR (DMSO-d 6 )0.79-0.83 (6H, m), 1.15 (1H, s), 1.29 (3H, s), 1.35-1.43 (4H, m), 1.78 (1H, s), 2.18 (3H, s), 1.91 (2H , s), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com