Oseltamivir warning structure impurity and preparation method thereof
A technology of oseltamivir and impurities, applied in the field of drug synthesis, can solve problems such as increasing the risk of safe drug use, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
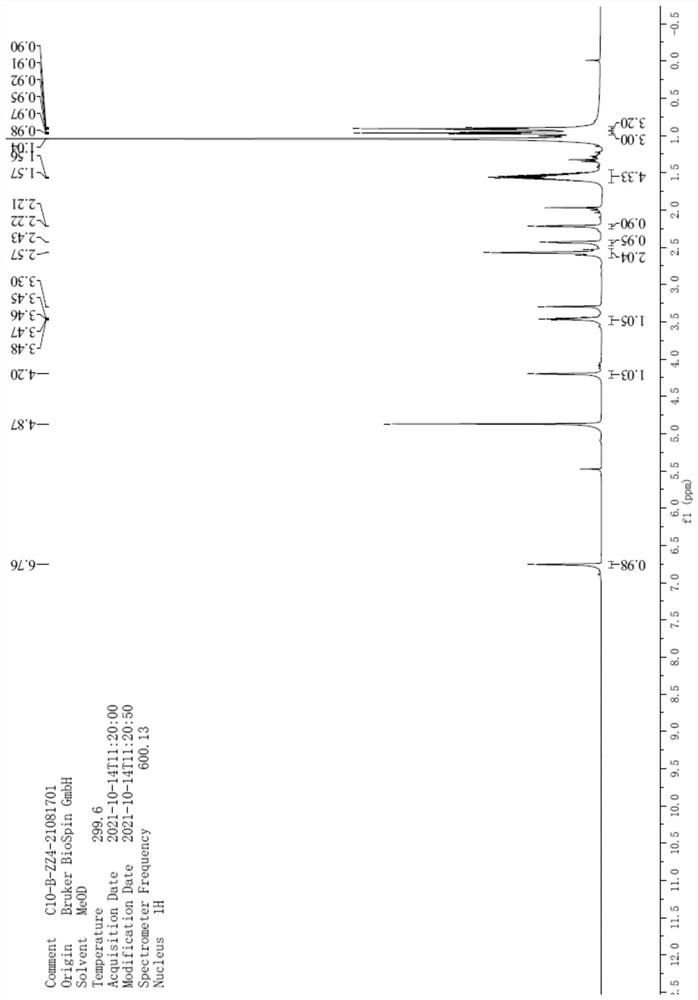
[0036] Example 1 Synthesis of Oseltamivir Warning Structure Impurity (I)
[0037] At room temperature, add 5.00 g of compound (II) (13.79 mmol), ethanol (50 mL) to the reaction flask, stir to dissolve, and cool to 0-10 ° C, add 1.1 g of sodium hydroxide (27.50 mmol) in 10 ml of aqueous solution, Stir overnight at 0-10°C, distill the reaction solution to dryness at 50°C under reduced pressure, add 10ml of dichloromethane and 10ml of water to the residue, add concentrated hydrochloric acid dropwise to adjust the pH=6-7, oily matter is precipitated, and the oily matter is separated Purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 10:1 (volume ratio), and 3.23 g of oseltamivir warning structure impurity (I) was obtained with a yield of 71.1% and a purity of 99.5%.
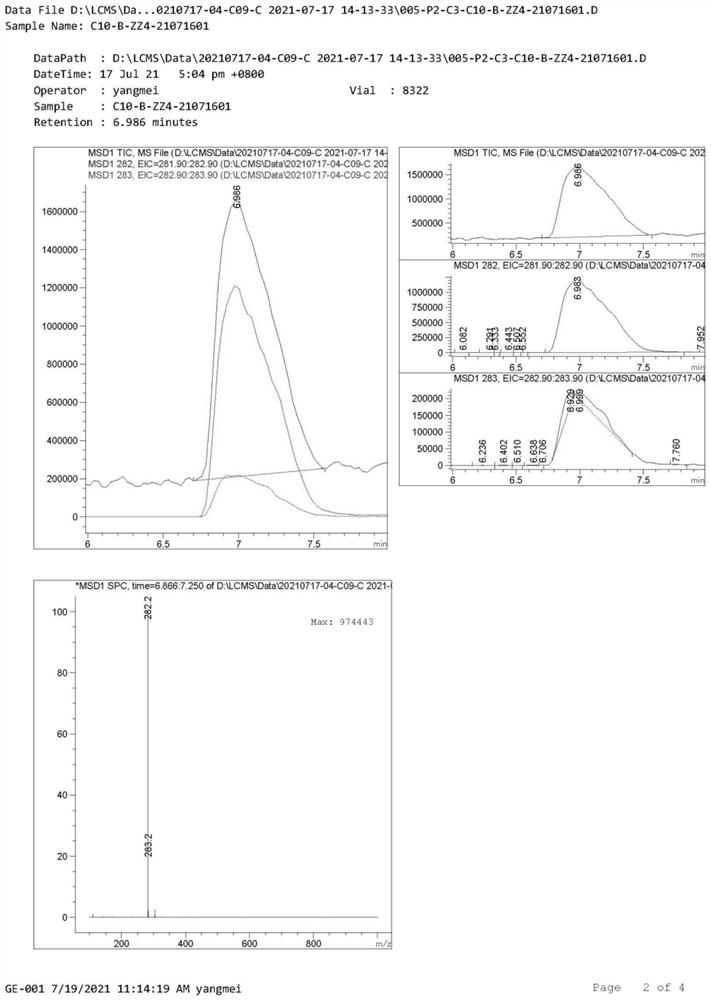
[0038] MS [M+H]: 282.2.
[0039] 1 H-NMR (600MHz, CD 3 OD) δ0.91(t, 3H), δ0.97(t, 3H), δ1.04(s, 6H), δ1.56(m, 4H), δ2.21(d, 1H), δ2.43 (d, 1H), δ2.57(t, 2H), δ3.46(m, 1H), δ4.20(s,...
Embodiment 2
[0040] Example 2 Synthesis of oseltamivir warning structure impurity (I)
[0041] At room temperature, add 5.00g of compound (II) (13.79mmol), methanol (50mL) to the reaction flask, stir to dissolve, and cool to 0-10°C, add 1.2g of potassium hydroxide (21.43mmol) in 20ml of aqueous solution, Stir overnight at 0-10°C, distill the reaction solution to dryness at 50°C under reduced pressure, add 10ml of dichloromethane and 10ml of water to the residue, add concentrated hydrochloric acid dropwise to adjust the pH=6-7, oily matter is precipitated, and the oily matter is separated Purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 10:1 (volume ratio), and 3.14 g of oseltamivir warning structure impurity (I) was obtained with a yield of 69.3% and a purity of 99.8%.
Embodiment 3
[0042] Example 3 The detection method of the oseltamivir warning structure impurity (I) in the product
[0043] Instruments and equipment: liquid chromatograph, electronic balance, volumetric flask
[0044] Chromatographic conditions:
[0045]
[0046]
[0047] Determination:
[0048] Blank solution: 50% acetonitrile
[0049] Reference substance solution: accurately weigh an appropriate amount of impurity (I) reference substance, dilute it with a blank solution to make about 10 μg / ml, and prepare 2 parts in parallel;
[0050] Test product solution: Accurately weigh an appropriate amount of oseltamivir test product, dilute it with a blank solution to make a 1mg / ml solution, and prepare 2 parts in parallel.
[0051] Quantitation limit: 0.05ug / ml
[0052] Detection limit: 0.017ug / ml
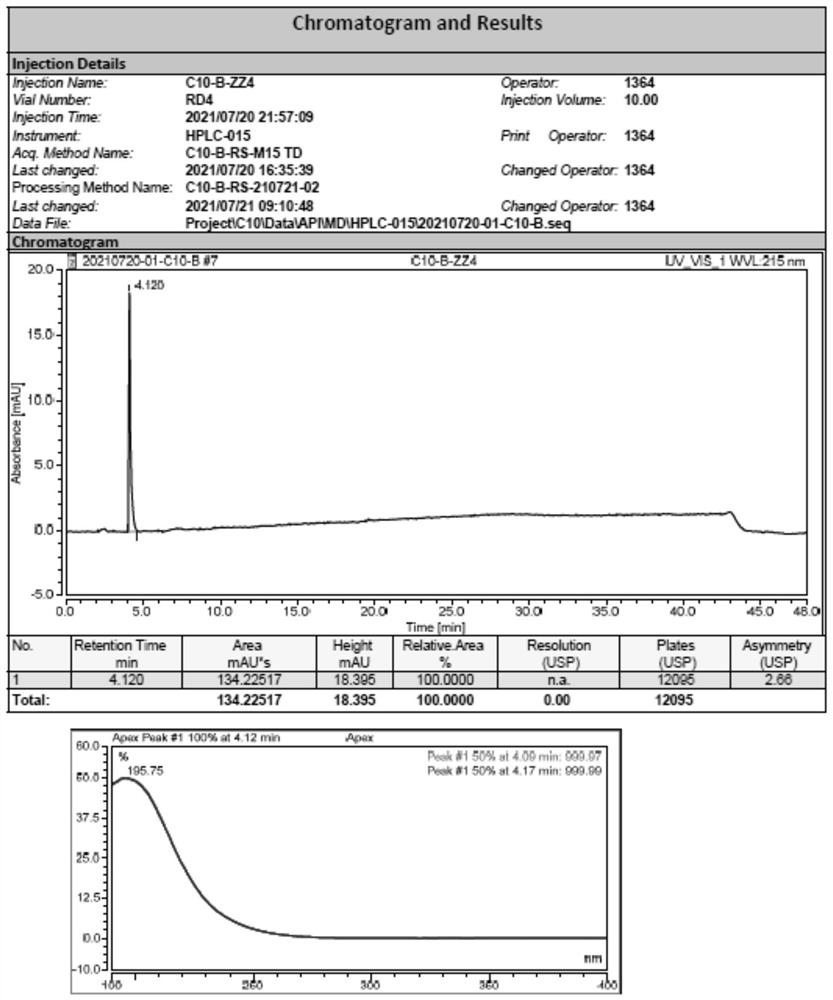
[0053] Sample detection result: lower than the detection limit (see the detection spectrum image 3 , Figure 4 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com