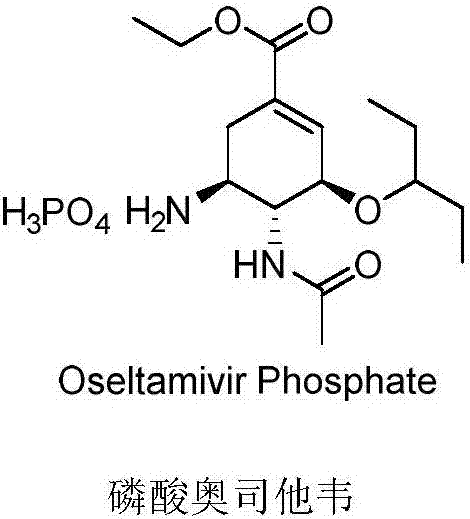
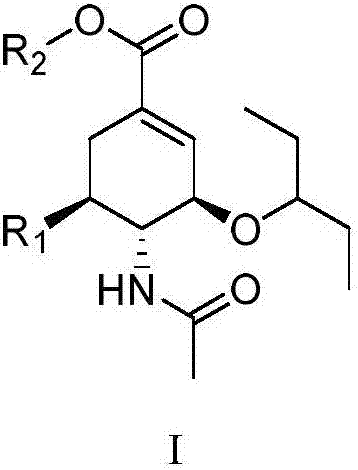
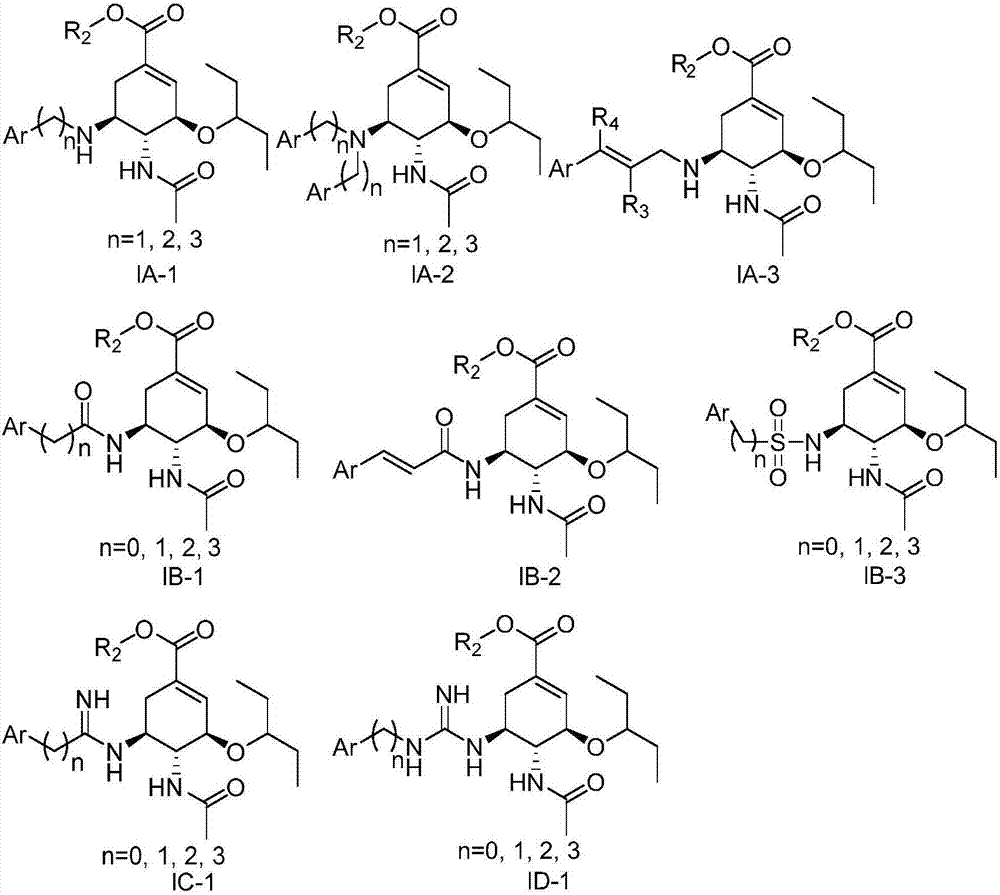
Oseltamivir derivative, preparation method and application thereof
A technology of oseltamivir and derivatives, which is applied in the fields of organic compound synthesis and medical application, and can solve problems such as inconvenience for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] (3R,4R,5S)-4-Acetamido-5-((4-nitrobenzyl)amino)-3-(pentane-3-oxyl)cyclohexene-1-ene-1-carboxy Preparation of ethyl acetate (1)
[0102]
[0103] Weigh oseltamivir phosphate (0.41g, 1mmol), 4-nitrobenzaldehyde (0.15g, 1.2mmol), sodium cyanoborohydride (0.12g, 2mmol) in 20mL ethanol solution, stir at 30°C for 5h (TLC detects that the reaction is complete, developer: ethyl acetate). Evaporate the solvent, add 20mL water, extract three times with 20mL ethyl acetate, dry over anhydrous magnesium sulfate, filter, evaporate the solvent, and perform flash column chromatography to obtain a white solid which is the compound (3R,4R,5S)-4- Ethyl acetamido-5-((4-nitrobenzyl)amino)-3-(pentane-3-oxy)cyclohexene-1-ene-1-carboxylate, yield: 76.3%.
[0104] (3R,4R,5S)-4-Acetamido-5-((4-nitrobenzyl)amino)-3-(pentane-3-oxyl)cyclohexene-1-ene-1-carboxy Preparation of acid (IA-1-1)
[0105]
[0106] (3R,4R,5S)-4-acetamido-5-((4-nitrobenzyl)amino)-3-(pentane-3-oxyl)cyclohexene-1-ene...
Embodiment 2
[0109] (3R,4R,5S)-4-Acetamido-5-((3-trifluoromethylbenzyl)amino)-3-(pentane-3-oxyl)cyclohexene-1-ene-1 - Preparation of ethyl carboxylate (2)
[0110]
[0111] The operating steps are the same as in Example 1, except that the starting material used is 3-trifluoromethylbenzaldehyde.
[0112] The product was obtained as a white solid, yield: 77.1%.
[0113] (3R,4R,5S)-4-Acetamido-5-((3-trifluoromethylbenzyl)amino)-3-(pentane-3-oxyl)cyclohexene-1-ene-1 - Preparation of ethyl carboxylate (IA-1-2)
[0114]
[0115] The operation steps are the same as in Example 1, except that compound 2 is used as the starting material.
[0116] The product was obtained as a white solid, yield: 79.3%.
[0117] 1 H NMR (400MHz, DMSO-d 6 )δ12.71(s,1H),9.90(s,1H),9.53(s,1H),8.22(d,J=9.0Hz,1H),7.99(s,1H),7.87(d,J=7.6 Hz, 1H), 7.76(d, J=7.8Hz, 1H), 7.66(t, J=7.7Hz, 1H), 6.66(s, 1H), 4.30(s, 3H), 4.05(q, J=8.9 Hz,1H),3.53–3.46(m,1H),2.96(dd,J=17.1,4.8Hz,1H),2.77–2.65(m,1H),1.91(s,3H),1.42(q...
Embodiment 3
[0119] Preparation of 4-(Benzo[b]thiophen-2-yl)benzaldehyde (3-1)
[0120]
[0121] Benzo[b]thiophene-2-boronic acid (1.78g, 10mmol), 4-bromobenzaldehyde (1.85g, 10mmol), potassium carbonate (4.1g, 30mmol), tetrakis(triphenylphosphine)palladium (0.08g , 0.069mol), DMSO (30mL) were added into the flask, under nitrogen protection, heated to 120°C, and stirred for 12 hours. The reaction mixture was added to 150 mL of water, extracted 3 times with 50 mL of ethyl acetate, dried over anhydrous magnesium sulfate, filtered, evaporated to remove the solvent, and separated by flash column chromatography to obtain a pale yellow oil which was the compound 4-(benzo[b] Thiophen-2-yl)benzaldehyde (3-1), yield: 82.5%.
[0122] (3R,4R,5S)-4-acetamido-5-((4-(benzo[b]thiophen-2-yl)benzyl)amino)-3-(pentane-3-oxyl) ring Preparation of ethyl hexene-1-ene-1-carboxylate (2)
[0123]
[0124] The operation steps are the same as in Example 1, except that the starting material is 4-(benzo[b]thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com